Ethylene C2h4 Lewis Structure
To do that we always count our valence electrons up first. Count total valence electron in C2H4.
7 3 Lewis Symbols And Structures Chemistry
In a double bond two pairs of valence electrons are shared for a total of four valence electrons.
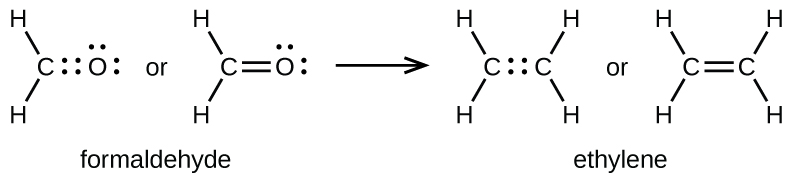
Ethylene c2h4 lewis structure. Be sure to include all resonance structures that satisfy the octet rule. It is a chemical formula for Ethylene or Ethene. Alternatively a dot method can be used to draw the lewis structure.
Ethene C 2 H 4. We have 12 available valence electrons. The key to understanding how to distribute the valence electrons is to.
Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence. Calculate the total valence electrons in the molecule. Ethenes lewis structure can be built by VSEPR rule.
Hybridization of atoms in ethene molecue can be found from lewis structure. Ethylene CH2CH2 or C2H4 CID 6325 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair.
However in Hydrocarbons we always place the Carbon atoms in the center as shown in the figure. 8C 4H 12 Valence Electrons. Carbon is in group 4 sometimes written 14 so it has 4 valence electrons.
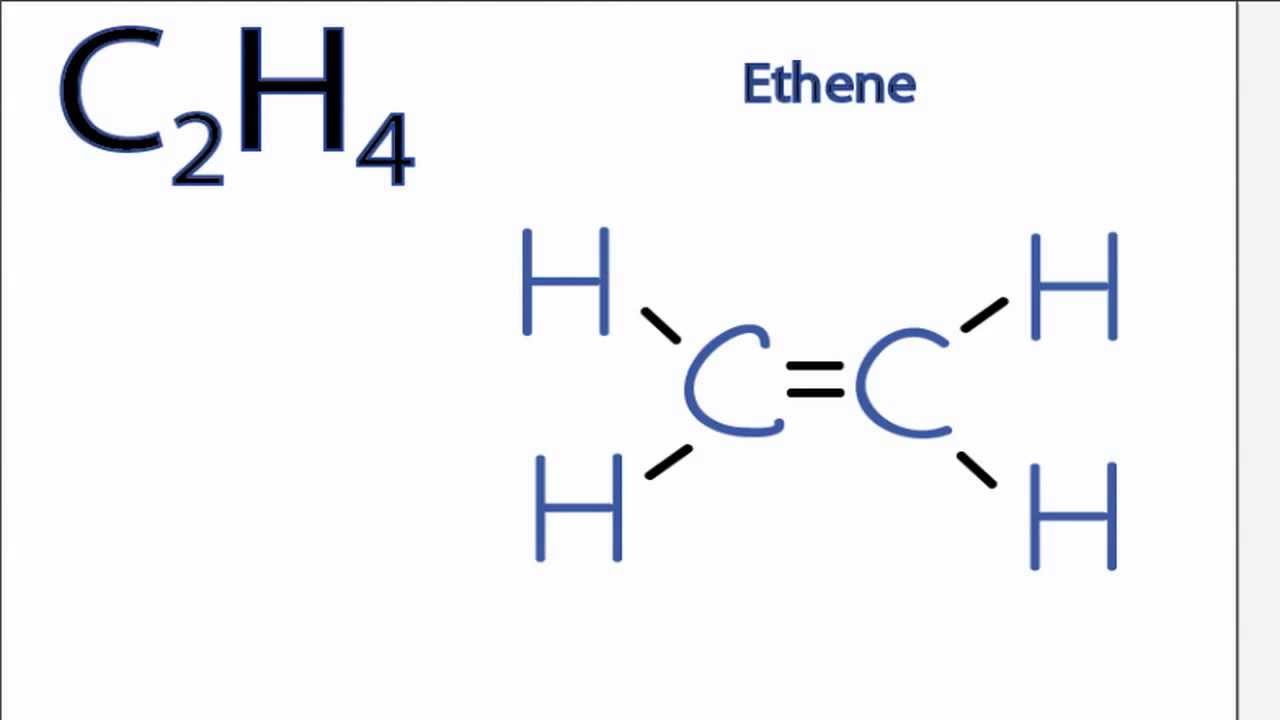
According to the VSEPR chart the shape of the ethene molecule is trigonal planar. There are two triangles overlapping each other as we can see in the diagram. Its C2H4 and we want to write the dot structures for ethene.
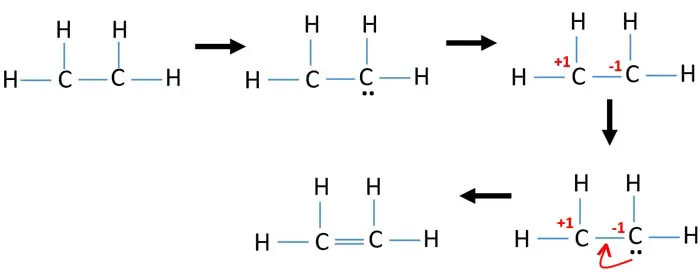
If we come way over here to Hydrogen its in group 1. Drawing the Lewis Structure for C 2 H 4. This means that the carbon atoms share 4 electrons.
Ortho-dichlorobenzene C6H4Cl2 is obtained when two of the adjacent hydrogen atoms in benzene are replaced with Cl. Drawing the Lewis structure for C 2 H 4 named ethene requires the use of a double bond. Draw the Lewis structure for the ethylene C2H4 molecule.
The electron dot structure is drawn using Lewis-dot structure. Structure properties spectra suppliers and links for. The lewis structure of C2H4 is very easy to draw-Some steps need to follow for drawing the C2H4 Lewis dot structure 1.
Each step of determining the lewis structure of ethene and hybridization are explained in this tutorial. It consists of two carbon molecules and 4 hydrogen molecules. Fill In The Orbital Diagram Of Unhybridized Valence.
It has double bond present between two carbon atoms. C2H4 Lewis structure contains four C-H bonds and one double bond in between two carbon atoms. C represents carbon atoms and H represents hydrogen atoms.
D The Lewis electron-dot diagram for C2H4 is shown below in the box on the left. Hydrogen is the least electronegative element here. Lets take a look.
Lewis dot structure of C 2 H 4. A Draw The Lewis Structure For Ethylene C2H4 B List The Electronic And Molecular Geometries For C2H4 Around The Central C Atom the C Atoms Are Equivalent. No lone pair is present on the central or outer atom in the lewis structure of ethene.
The Lewis structure of C2 H4 also known as ethene has two carbons with a double bond between them. C2H4 Lewis Structure Ethylene Welcome to Geometry of Molecules and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure of C2H4 molecule. Ethene C 2 H 4 Lewis Structure Hybridization.
It has 1 valence electron. Welcome to Geometry of Molecules and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure. Draw the Lewis structure for ethylene C2H4.
Ethene is an unsaturated hydrocarbon. What Is The Hybridization Of C In Ethylene. Experts are tested by Chegg as specialists in their subject area.
Answered by ExpertThe Lewis structure for C2H4 consists of two C symbols connected by two lines. For C 2 H 4 you have a total of 12 total valence electrons. Each C symbol is connected to two H symbols with a single line for each.
We review their content and use your feedback to keep the quality high. Electron Dot Structure for ethane C2H4. Most stable structure is taken as the lewis structure of ethene.
Use information from step 4 and 5 to draw the lewis structure. Therefore the total number of valence electrons in Ethylene C 2 H 4.

Draw The Lewis Structure For The Ethylene C2h4 Chegg Com

Draw The Electron Dot Structure Of Ethene C2h4 Brainly In

Structure And Bonding In Ethene The Pi Bond Chemistry Libretexts

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Ethene C2h4 Lewis Structure Hybridization
Is C2h4 Polar Or Nonpolar Youtube
Ethene C2h4 Lewis Structure Hybridization

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
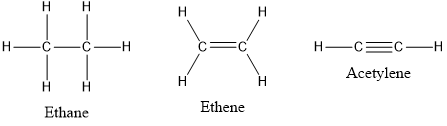
Solved A Draw Lewis Structures For Ethane C2h6 Ethylene C2h Chegg Com

Write The Electron Dot Structure Of Ethene Molecule C2h4
Lewis Structure Of C2h4 Biochemhelp

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
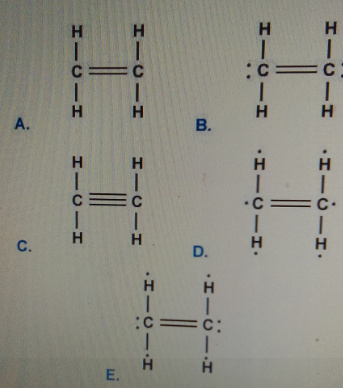
Which Is The Correct Lewis Structure For Ethylene C2h4 Home Work Help Learn Cbse Forum

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar
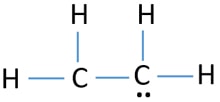
Ethene C2h4 Lewis Structure Hybridization
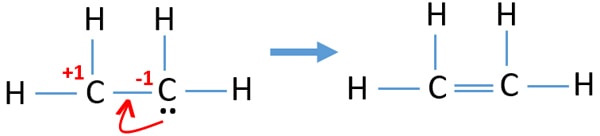
Ethene C2h4 Lewis Structure Hybridization

Draw The Lewis Structure For Ethylene C2h4 Be Certain You Clutch Prep
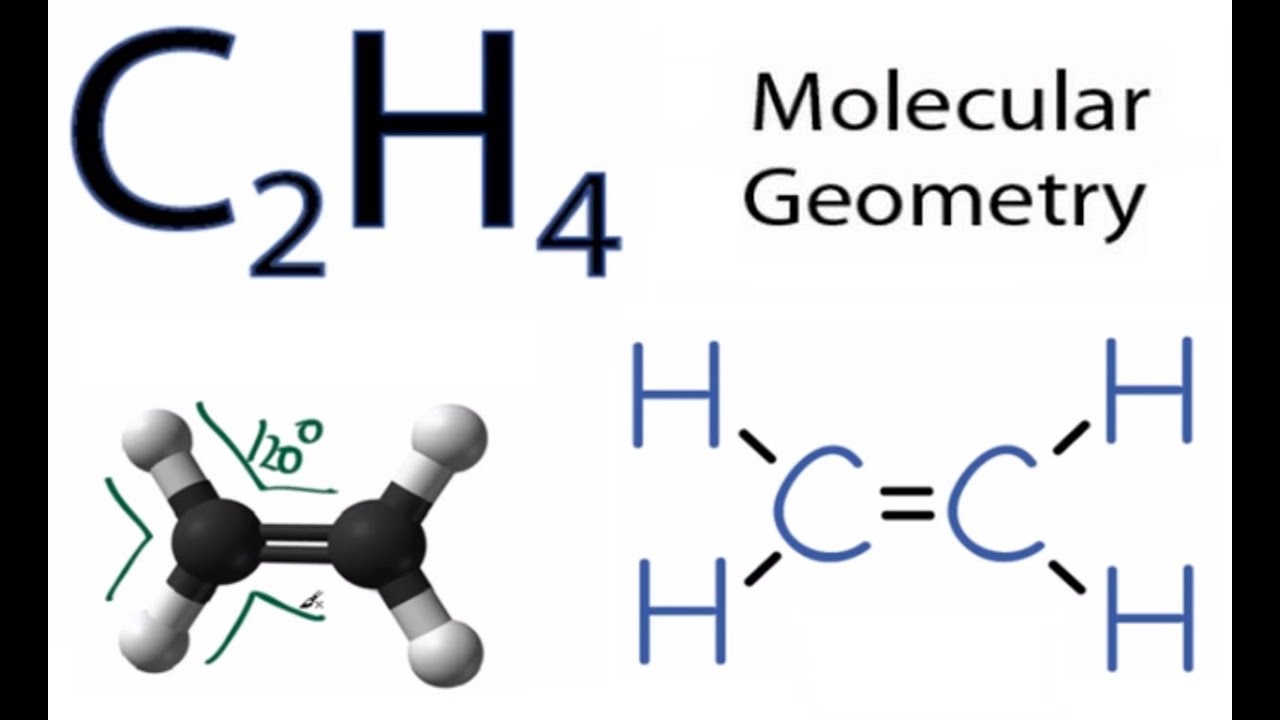
C2h4 Molecular Geometry Shape And Bond Angles Youtube

Draw The Lewis Structure For Ethylene C2h Clutch Prep