Hno3 Lewis Structure Hydrogen
Include all three resonance structures by alternating the double bond among the three oxygen atoms. Be sure to use the number of available valence electrons you found earlier.

Nitric Acid Hno3 Lewis Structure Properties Uses Rankred
That takes care of the H.

Hno3 lewis structure hydrogen. After determining how many valence electrons there are in HNO3 place them around the central atom to complete the octets. Nitric acid Lewis Structure. The Lewis Structure Lewis Dot Diagram for HCN1.
This chemistry video tutorial explains how to draw the lewis structure of HNO3 - Nitric AcidMy Website. Hydrogen will always have a total of two electrons from its one bond. In the Lewis structure we see that hypochlorous acid has 14 valence electrons.
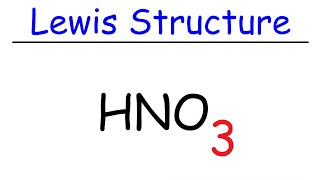
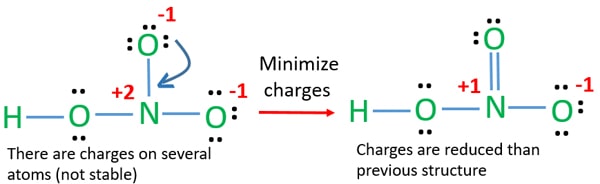
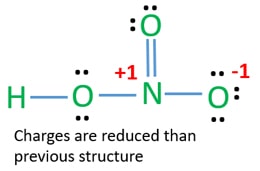
Include all three resonance hybrids by alternating the double bond among the three oxygen atoms. However the first two resonance structures are significantly more favorable than the third because they have smaller amount of formal charges. The HNO3 Lewis structure is best thought of as the NO3 with an H attache.
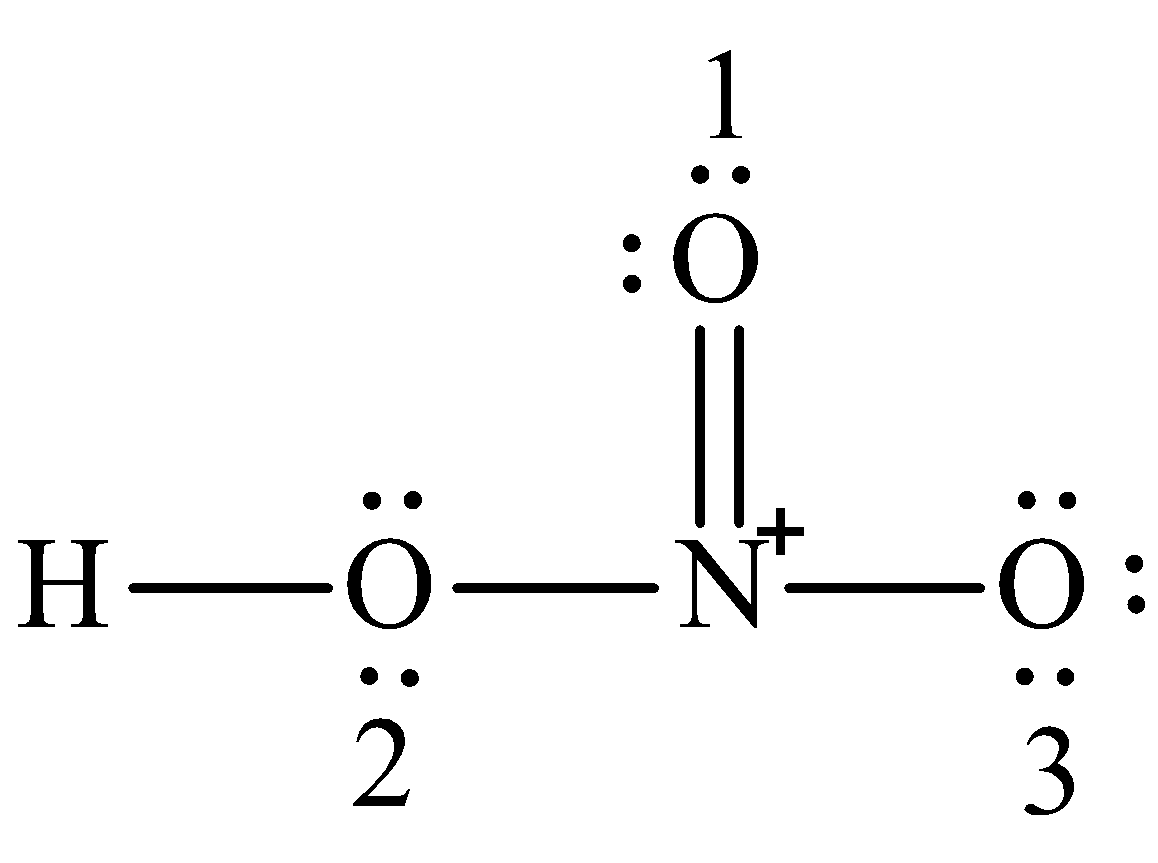
For the HNO3 Lewis structure calculate the total number of valence electrons for the HNO3 molecule. The nitrogen in HNO3 has a formal 1 charge and a formal double bond to one of its oxygens. Put one electron pair in each bond4.
This is a pattern seen with many acids. The N atom in HNO3 has SP2 hybridization and the O atom has SP3 hybridization. The HNO3 Lewis structure is best thought of as the NO3 with an H attached to one of the oxygen atoms.
Draw the best Lewis structure for HNO 3 Nitrogen is the center atom hydrogen is connected to one of the Oxygen atoms. A step-by-step explanation of how to draw the HNO3 Lewis Structure Nitric Acid. In reality the bond order is more complex But the nitrogen can accept an electron pair donating its formal pi bond to the oxygen that is formally double bonded to it.
Draw molecules by placing atoms on the grid and connecting them with bonds. Beside above what is the shape of Hocl. For HNO3 in order to satisfy the octet rule the nitrogen atom would form 1 double bond and 2 single bonds.
Include all nonbonding electrons. Draw a Lewis structure for nitric acid HNO3 the hydrogen atom is attached to one of the oxygen atoms. Boron can have fewer than eight electrons but never more than eight.
Nitrogen has a steric number of 3 while the oxygen atom in the OH ion has a steric number of 4. Is co3 2 a resonance structure. When we see an H in front of a polyatomic structure like the CO3- here that means the H will be attached on the outside of one of the Oxygens.
In a reasonable Lewis structure carbon nitrogen oxygen and fluorine always have eight electrons around them. Use formal charge to determine which of the resonance structures is most important to the structure of nitric acid. Four are used as bonding electrons and the remaining.
Put least electronegative atom in centre3. Based on octet rule alone there are 3 possible resonance structures that are favorable. The HNO3 Lewis structure has 24 valence electrons.
In the Lewis structure of HNO3 the molecule is formed due to the hybridization of two orbitals. Lewis acids accept electron pairs. Draw the Lewis structure for nitric acid the hydrogen atom is attached to one of the oxygen atoms.
In the lewis structure of nitric acid there is a 1 charge on nitrogen atom and one double bond between nitrogen and one oxygen atom. For molecules with resonance each lewis structure individually does not accurately depict the structure of the molecule. The Lewis structure of hypochlorous acid has oxygen O with single bonds between hydrogen and chlorine.
For HCO3- we have a total of 24 valence electrons. A molecule has resonance if more than one lewis structure can be drawn for that molecule. Drawing the Lewis Structure for HNO After determining how many valence electrons there are in HNO3 place them around the central atom to complete the octets.
Unlike O3 though the actual structure of CO32 is an average of three resonance structures. So well put Carbon at the center and then well put an OH over here. Each atom except hydrogen and boron.
HNO 3 Nitric acid lewis stricture is drawn step by step by using valence electrons of each element.

Consider The Lewis Structure For The Nitri Clutch Prep

Ch2cl2 Lewis Structure Dichloromethane In 2021 Molecules Math Equations Hydrogen Atom

Draw The Lewis Structure For Hno3 And State Its Molecular Geometry Is It Polar Or Nonpolar Study Com

Pcl3 Lewis Structure Phosphorus Trichloride In 2021 Math Equations Lewis Chemical Formula

Pcl3 Lewis Structure Phosphorus Trichloride In 2021 Math Equations Lewis Chemical Formula
Hno3 Lewis Structure Nitric Acid Youtube
Hno3 Nitric Acid Lewis Structure

Hno3 Lewis Structure Nitric Acid Youtube

Draw A Lewis Structure For Nitric Acid The Hydrogen Atom Is Attached To One Of The Oxygen Atoms Brainly Com

Hno3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Hno3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Hno3 Nitric Acid Lewis Structure

1 Draw The Lewis Structure Of Nitric Acid Hno 3 That Minimizes Formal Charges Assign Lone Pairs Radical Electrons And Atomic Charges Where Appropriate 2 Calculate The Electrons Required Er Study Com

How To Draw A Lewis Structure Lewis Chemistry Draw

Consider The Lewis Structure For The Nitri Clutch Prep

Easy Steps To Draw Lewis Structure Of Hno3 Nitric Acid Youtube

How Is The Lewis Dot Structure For Nitric Acid Determined Quora
Write Lewis Structure Of The Hno3 And Show Formal Charge Class 12 Chemistry Cbse
