How Many Lone Pairs In Sih4
What is the Lewis structure of SiH4. By signing up youll get thousands of.

Sih4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
Do not include the formal charges.

How many lone pairs in sih4. If the number is 4 there are two lone pairs on the central atom. The Lewis dot structure for SiH4 possesses how many lone pairs of electrons around the central atom. How many double bonds are there.
Exactly one unshared pair of valence electrons SiH4 H2S PH3 PH3 How many total bonds and lone pairs exist in the Lewis structure for boron trichloride BCl3 Lone pair Wikipedia April 18th 2019 - In chemistry a lone pair refers to a pair of valence electrons that are not shared with another atom and is sometimes called an unshared pair. There are no valence electrons left over so the molecule has four bond pairs and no lone pairs. Thus the total number of remaining electrons is 26.
Draw the Lewis structure of SiH_4 including lone pairs. Draw the Lewis structure of the following molecule. How many total bonds and lone pairs exist in the Lewis structure for boron trichloride BCl3.
If the number is 6 there are three lone pairs on the central atom. Draw Lewis structures for the following xenon compounds. A step-by-step explanation of how to draw the SiH4 Lewis Dot Structure Silicon TetrahydrideFor the SiH4 structure use the periodic table to find the total.
Why is SeCl4 polar. To do so we first need to do the following steps. How many total bonds and lone pairs exist in the Lewis structure for chlorine fluoride ClF.
Draw the Lewis structures of the following molecules. Draw the Lewis structure of NH3. Were being asked to draw a Lewis structure for SiH 4.
OF2 and CO2 3. So 3 2 5 also determines sp3d hybridization. Each hydrogen atom uses its valence electron to pair up with one of the Si valence electrons to form a SiH bond four of them in all.
Silanes refers to many binary silicon-hydrogen compounds and compounds with four substituents on silicon including. Silicon is in Group IVA or Group 14 of the Periodic Table so it has 4 valence electrons its electron configuration is 1s22s22p23s23p2 while hydrogen has one. Structure lewis structure of sih4 notice that there are 4 single bond how many total bonds and lone pairs exist in the lewis structure for chlorine fluoride clf 1 6 which of the following compounds contains exactly one unshared pair of valence.
Draw a Lewis structure of CH 3NH2. SO42-This problem has been solved. How many lone pairs are on the central atom in BCl 3.
Include lone pairs if necessary. If the number is 0 there are no lone pairs on the central atom. There are three iodine atoms one of which is also negatively charged.
How many lone pairs are on the central atom of B. If the number is 2 there is one lone pair on the central atom. The shape of the I3 molecule is linear.
Express your answer numerically as an integer. How many lone pairs does the sulfur atom possess in the hydrogen sulfide molecule. Let us now move to SF4s hybridization after we have seen its Lewis Structure.
Sulfur will have 2 valence electrons or 1 lone pair and 4 bonds. How many dots should be drawn in the Lewis Dot Structure of SiH4. How many dots should be drawn in the Lewis Dot Structure of P2S2.
Show all lone pairs. The number of lone pairs of electrons in this molecule is 3 and the number of atoms with valence electrons is 2. Does sih4 have a lone pair.
Silane is an inorganic compound with chemical formula Si H 4 making it a group 14 hydrideIt is a colourless pyrophoric toxic gas with a sharp repulsive smell somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Each Fluorine atom will have 3 lone pairs and 1 bond.
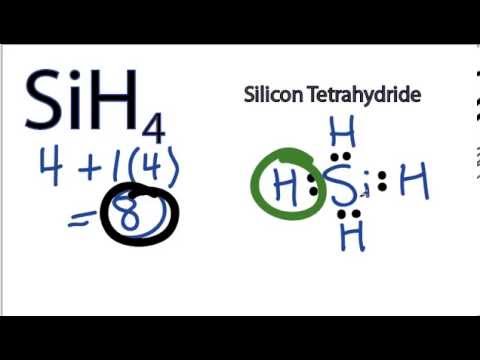
Each hydrogen atom uses its valence electron to pair up with one of the Si valence electrons to form a SiH bond four of them in all. There are no valence electrons left over so the molecule has four bond pairs and no lone pairs. Draw the Lewis structure for SiH4.
How many dots should be drawn in the Lewis Dot Structure of H2Se. Here in SiH4 A stands for Silicon Si X stands for the four Hydrogen atoms n 4 E stands for no lone pairs on Si x 0. Which of the following compounds contains exactly one unshared pair of valence electrons.
How many lone pairs are there. Represent shared electron pairs with dashes.

Sih4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
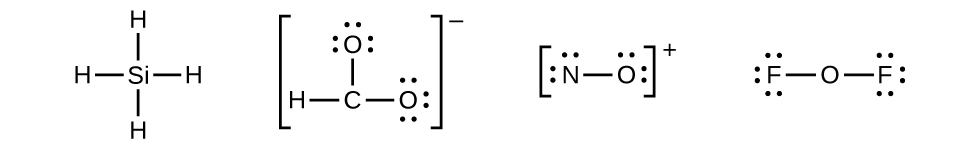
10 1 Lewis Structures And The Octet Rule Chemistry Libretexts

Vsepr For 4 Electron Clouds Video Vsepr Khan Academy
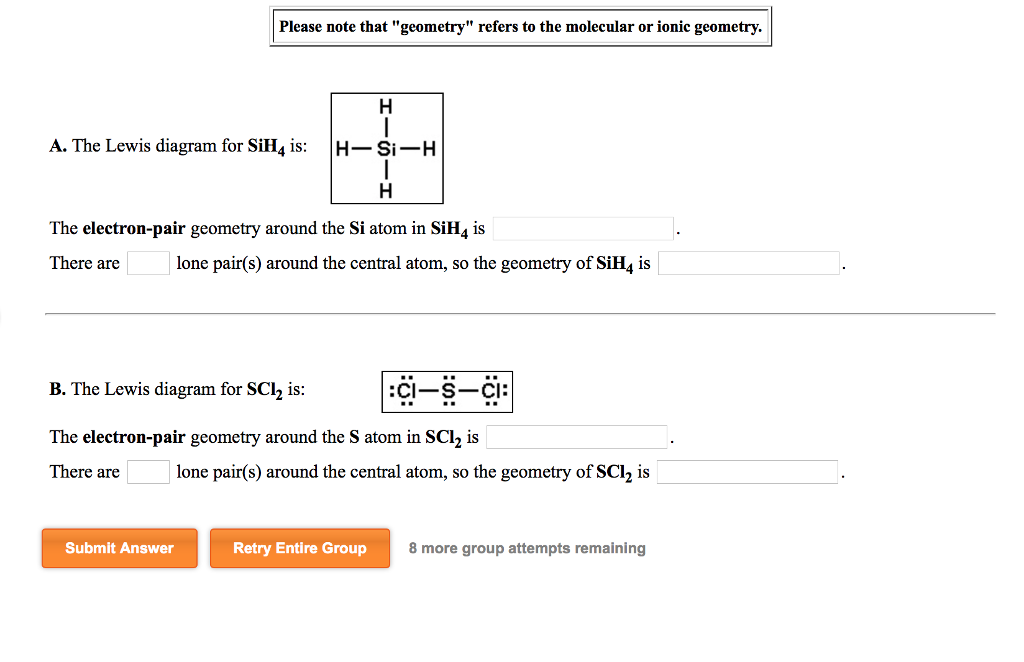
Please Note That Geometry Refers To The Molecular Chegg Com

Sih4 Lewis Structure How To Draw The Lewis Structure For Sih4 Silicon Tetrahydride Youtube

How Many Bonding Pairs And Lone Pairs Are There In Sih4 Quora

Write A Lewis Structure For Each Molecule Sih4 Draw The Mo Clutch Prep

Draw The Lewis Structure For Sih4 Clutch Prep
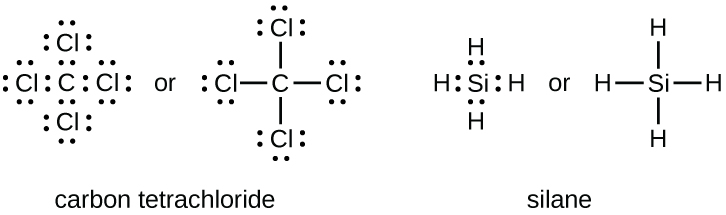
4 2 Lewis Structures Chemistry Libretexts

Which Of The Following Contains Exactly 2 Unshared Pairs Of Clutch Prep
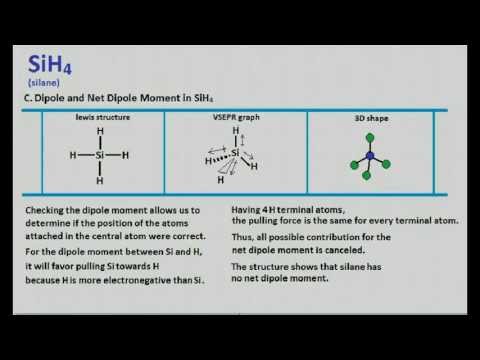
Sih4 Lewis Structure Molecular Geometry Youtube

Sih4 Lewis Structure Molecular Geometry Youtube

Complete The Following Chart For Sih4 Clutch Prep
Solved Draw A Lewis Structure For Sih4 What Is The Valence Bond Analysis Hybridization Of Si And H And Therefore What Is The Vb Analysis For T Course Hero

Sih4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Sih4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Sih4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
Sih4 Lewis Structure How To Draw The Lewis Structure For Sih4 Silicon Tetrahydride Youtube
