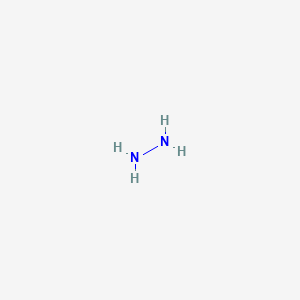
Hydrazine N2h4 Lewis Structure
The first picture the picture of the Lewis Structure of Hydrazine was accurate and very unique to the blog. In the N2H4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the ou Weve used six eight ten.
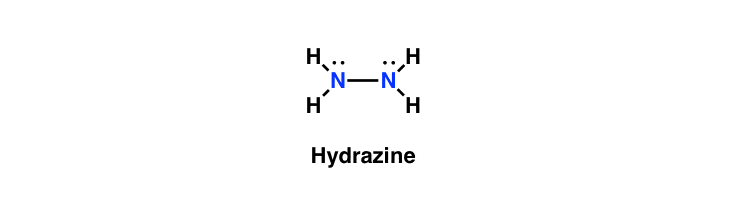
Reagent Friday Hydrazine Nh2nh2 Master Organic Chemistry
A stepbystep explanation of how to draw the n2h4 lewis structure dinitrogen tetrahydride or hydrizine.

Hydrazine n2h4 lewis structure. Asked Sep 3 2019 in Chemistry by Logic. Labels on household cleansers caution against mixing bleach with ammonia because the reaction produces monochloramine NH2Cl and hydrazine N2H4 both of which are toxic. Calculate the enthalpy change per mole of hydrazine combusted.
The exception of course being the hydrogens. Hydrazine N2H4 or H2NNH2 or H2N-NH2 or H4N2 CID 9321 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Hydrazine N2H4 is a liquid used as a rocket fuel.
Back in the center twelve and fourteen. Draw the Lewis structure of C₂H₄Cl₂ both Cl atoms on one C atom and then determine if the molecule is polar or nonpolar. Hydrogen H only needs two valence electrons to have a full outer shell.
In the Lewis structure for N2H4 there are a total of 14 valence electrons. H2-N-N-H2 with lone pairs on the nitrogen atoms each nitrogen has 2 hydrogens and a nitrogen attached. Each nitrogen atom has five valence electrons out of which there are three unpaired electron.
Hydrazine N2H4 is a good reducing agent that has been used as a component in rocket fuels. A chemist wanted to prepare hydrazine N2H4 a type of rocket fuel by the reaction. A H N N H H H B H N N H H H C H N N.
The somewhat darker turquoise gave the blog a sleek and simple look which I really liked. Select its Lewis structure. Include all nonbonding electrons such as lone pairs and any nonzero formal charges.
Draw the Lewis structure for hydrazine which has the formula N2H4. Chemistry learning made easy. Hybridization in the Best Lewis Structure.
Select its Lewis structure. Lewis Dot of Hydrazine. Lets do the N2H4 Lewis structure.
It melts at 193K and has a molar mass of 3003 gmol. Lewis symbol of hydrogen is. Also the application of air or H2O2 leads to oxidation of hydrazine N2H4 a colorless flammable liquid which results in the formation of N2H2.
Since all the atoms are in either period 1 or 2 this molecule will adhere to the octet rule. The correct lewis structure for Hydrazine N2H4 is. N2H4 is straightforward with no double or triple bonds.
Forms a colorless gas and has an ammonia like smell. Next was the 3-D structure. Below is the chemical equation of preparation of Dinitrogen DihydrideDiazeneDiimide NCOOH2 NH2 2 CO2.
N2H4l O2g N2g 2 H2Ol The reaction of 650 g N2H4 evolves 1262 kJ of heat. Lewis structure of rocket fuel hydrazine. For structure calculate total number valence elect.
Best Lewis Structure The Lewis structure that is closest to your structure is determined. In the N2H4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside. Lewis symbol of nitrogen is.
First week only 499. Start your trial now. 2NH3 OCl- N2H4 Cl- H2O which essentially goes to completion.
It reacts with oxygen to yield nitrogen gas and water. Select its Lewis structure. Both nitrogen atoms form a single covalent bond by a sharing of two unpaired electrons.
Most blogs that I had looked at did not have the Lewis Structure. The hybridization of the atoms in this idealized Lewis structure is given in the table below. I quickly take you through how to draw the Lewis Structure of NH2NH2 Hydrazine.
Why cant it be H2-N- triple bond-N-H2 with the nitrogens tripled bonded together and then with 2 Hydrogens attached to each. Is a good reducing agent that has been used as a component in rockets fuels. Hydrazine N2H4 is a good reducing agent that has been used as a component in rocket fuels.
Hydrogen only needs two for a full outer shell so all of our Hydrogens are OK. Well determine the n2h4 molecular geometry with respect to nitrogen on right the other atom will have same shape since they are symmet. A bonding orbital for N1-N2 with 19954 electrons __has 4999 N 1 character in a sp282 hybrid.
H-NEN-H A H-NEN-H B HH н. 70 More Lewis Dot Structures. They follow the duet rule 2 electrons.

The Lewis Structure Of Hydrazine Youtube
Why Is Hydrazine N2h4 Polar It Seems To Me That The Sum Of The Left Side S Dipole Moments And The Right Side S Dipole Moments Are In Opposite Directions And Would Cancel Out
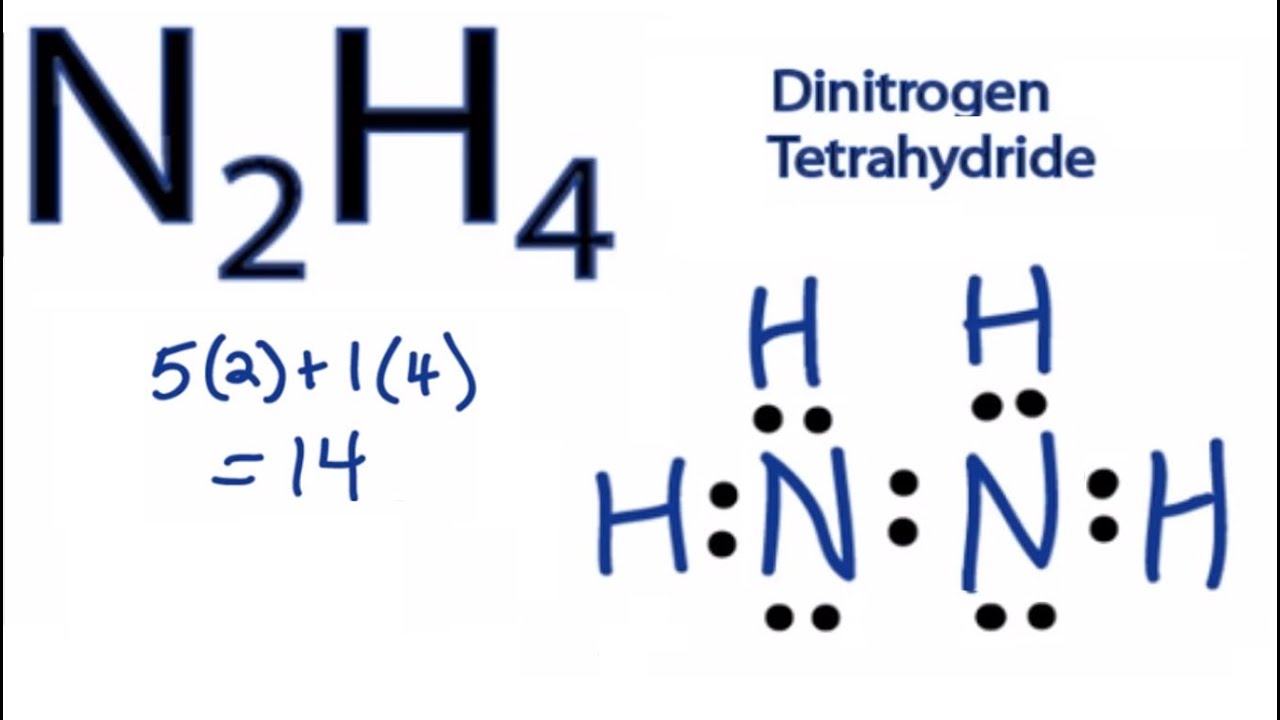
N2h4 Lewis Structure How To Draw The Lewis Structure For N2h4 Youtube

Nh2nh2 Lewis Structure How To Draw The Lewis Structure For Hydrazine Youtube
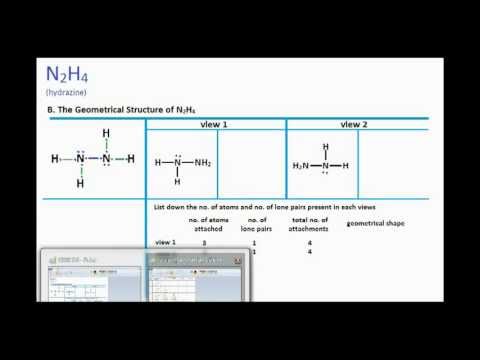
N2h4 Lewis Structure And Molecular Geometry Youtube

Hydrazine N2h4 And Carbon Disulfide Cs2 Clutch Prep

Hydrazine N2h4 Structure Molecular Mass Properties Uses

Chem Ch 9 11 Flashcards Quizlet
Why Does N N Dimethylhydrazine Have Hydrazine When It Has N2h2 Not N2h4 Hydrazine Why Isn T It Called Dimethyldiimide Quora

Hydrazine N2h4 Lewis Structure
What Is The Lewis Dot Structure For N2h4 How Is It Made Quora

Hydrazine N2h4 Is A Good Reducing Agent That Has Chegg Com
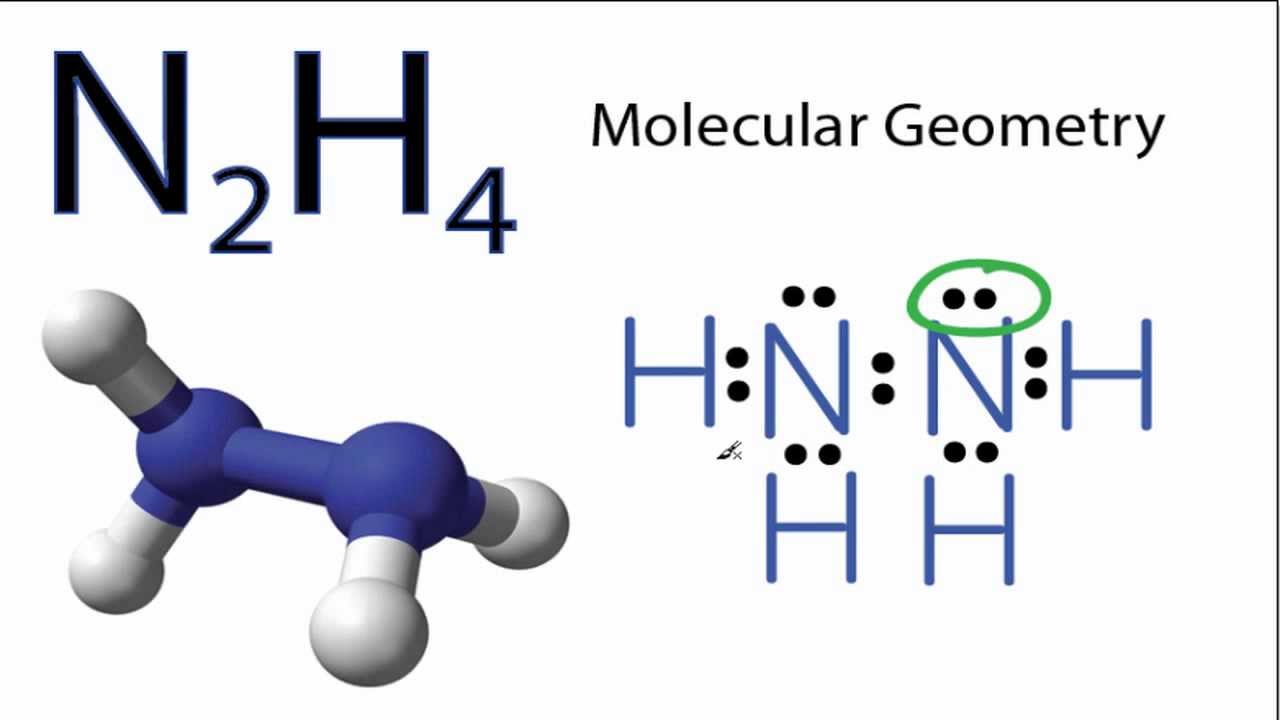
N2h4 Molecular Geometry And Bond Angles Actual Bond Angle Is Less Than 109 5 Degrees Youtube

Hydrazine N2h4 Hydrazine Polar Molecule
What Is The Lewis Dot Structure For N2h4 How Is It Made Quora
What Is The Lewis Dot Structure For N2h4 How Is It Made Quora

What Is The Lewis Structure For N2h4 Study Com