Is Xef2 Polar
Polar molecules interact strongly with other polar molecules but do not interact with nonpolar molecules and vice verse. F-Xe-F and 3 paired-electrons groups.

Pf5 Molecular Geometry Shape And Bond Angles Molecular Geometry Geometry Shape Molecular
The Xe-F bonds are polar as the electronegativity difference between xenon 26 and fluorine 40 is 14.

Is xef2 polar. It is colorless in appearance and has a characteristic odor. XeF2 is a nonpolar molecule despite two Xe-F bonds are polar. So XeF2 will be Raman active only.
Is SO2Cl2 Polar Or Nonpolar. The electronegativity of Xe is 26. XeF2 is a linear molecule due to the arrangement of fluorine atoms and the lone pairs of electrons in the symmetric arrangement.
But we know molecular polarity is a vector quantity which means net polarity is the vector sum of individual bond dipoles. The polarity induced on. First week only 499.
Not necessarily possess polrity. Hey Guys In this video we are going to determine the polarity of Xenon Difluoride having a chemical formula of XeF2. OF2 Oxygen difluoride is polar in nature because of its bent shaped geometrical structure and difference between the electronegativity of Oxygen and Fluorine atoms.
In chemistry polarity is defined as a physical property of matter relating to the unequal dispersion of partial charges between intermolecular atoms. It has a symectrical bonding so it can be considered nonpolar just on. As a result the dipole moment of Acetone is around 269 D.
XeF4 is a nonpolar molecule despite four individual Xe-F bonds are polar. Detailed Explanation As we have already known there are certain conditions for a molecule to be polar or nonpolar in the above definitions of the polar. Chemistry QA Library Is the molecule XeF2 polar or nonpolar.
What kind of bond is XeF4. The XeF2 has a linear molecular geometry and Xe-F bonds are symmetrical to each other as a result the net dipole moment becomes zero. CO2 By signing up youll get thousands of step-by-step.
Start your trial now. The central atom of XeF2 Xe has a total of 10 electrons localized in the form of 4 as Xe-F bonding and 6 as non-bonding pairs. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms.
For a molecule to be Raman active it must undergo a change in polarizability during excitation. Which of the following is a polar molecule. Chemistry questions and answers.
This is because XeF4 has an octahedral symmetric geometry. It is made of one Xenon atom and two Fl. The 2 bond groups form a 180 degree bond due to.
Question Is XeF2 polar or nonpolar. Has a formula AX2E2 and is linear. XeF2 is nonpolar in nature because of its linear-shaped geometry having fluorine atoms symmetrically on both sides of the xenon atom.
The compound XeF2. Why is XeF2 a linear structure. As a result the dipole moment of the molecule turns out to be nonzero making the OF2 a polar.
Acetone exists in a liquid state at room temperature. Is NOCI Polar Or Nonpolar. Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment.
Answer XeF2 Xenon difluoride is Nonpolar What is polar and non-polar. It is non-polar. In the periodic table fluorine F atom has the highest electronegativity value 40 and francium Fr has the lowest 07.
Is the molecule XeF2 polar or nonpolar. However the nonpolar molecules are more attracted to themselves than they are to the polar water molecules. However Xe-F bond is polar because the electronegativity of Xe and F is different but the polarity of both Xe-F bonds gets canceled by each other resulting in a nonpolar XeF2 molecule.
Is XeF2 Polar Or Nonpolar. So the shape of XeF2 ion is linear. Why is XeF2 a nonpolar molecule.
In the XeF2 compound the electronegativity difference between fluorine 398 and Xe 26 is 398-26 138 which shows the Xe-F bond is polar according to. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. The molecular structure of xenon tetrafluoride is square planar with six uniformly placed sp 3 d 2 orbitals forming 90-degree angles.
The electronegativity of Xe is 26. In the XeF2 compound the electronegativity difference between fluorine 398 and Xe 26 is 398-26 138 which shows the Xe-F bond is polar according to the Pauli scale.

Is Cs2 Polar Or Nonpolar Carbon Disulfide In 2021 Math Equations Chemical Formula Molecules

Xef2 Structure All Knowledge About Xef2 Molecular Geometry Knowledge Vsepr Theory

Is Pf5 Polar Or Non Polar Phosphorus Pentafluoride In 2021 Chemical Formula Molecules Phosphorus

Is Sf4 Polar Or Non Polar Sulfur Tetrafluoride In 2021 Math Equations Chemical Formula Molecules

Is No3 Polar Or Nonpolar Nitrate In 2021 How To Find Out Molecules Polar

Hybridization Of Ch3cl Chloromethane In 2021 Molecules Lewis Chemical Formula

Xef2 Lewis Structure Xenon Difluoride In 2021 Lewis Molecules Math Equations

Becl2 Lewis Structure Beryllium Chloride In 2021 Math Equations Lewis Molecules

Xef2 Lewis Structure How To Draw The Lewis Structure For Xef2 In 2021 Lewis Molecules Math Equations

Is Cn Polar Or Non Polar Cyanide In 2021 Chemical Chemical Formula Polar

Is Ch3oh Polar Or Nonpolar Methanol In 2021 Functional Group Molecules Chemical Formula

Xef2 Structure All Knowledge About Xef2 Molecular Geometry Knowledge Vsepr Theory
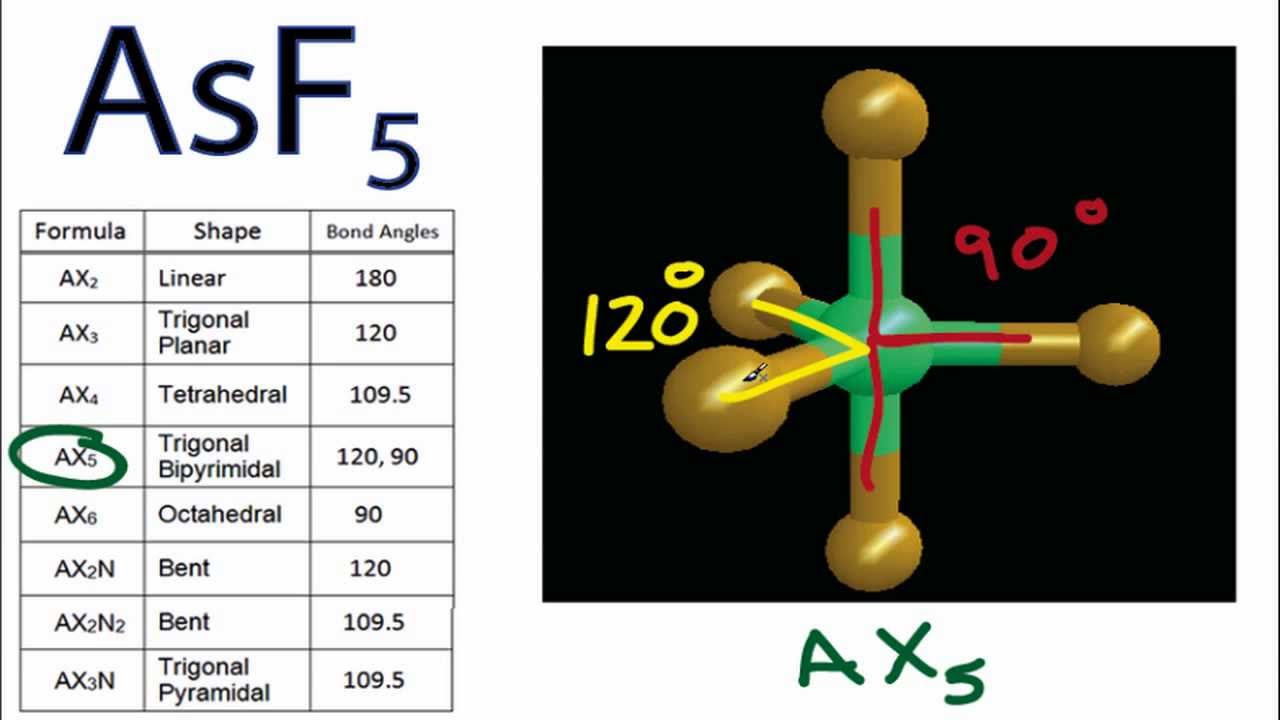
Asf5 Molecular Geometry And Bond Angles Arsenic Pentafluoride Molecular Geometry Molecular Geometry

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar

Is Chf3 Polar Or Nonpolar Fluoroform In 2021 How To Find Out Molecules Hydrogen Atom

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar

Is Nh2 Polar Or Non Polar Amide Ion In 2021 Nh 2 Molecules Electrons

