Lewis Diagram Of C2h2
Valence electrons of Carbon atom Valence electrons of Carbon atom. Acetylene has been detected 12 29 and 50 of total hydrocarbon concentration in emissions from vehicle exhaust petroleum exhaust and petrochemical plants respectively 2.

C2h2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist
Is C2H2 a resonance.

Lewis diagram of c2h2. The solution is to share three pairs of valence electrons and form a triple bond between the Carbon atoms in C 2 H 2. The Lewis structure of C 2 H 2 is. C2H2 the C atoms are f.
See the answer See the answer See the answer done loading. This problem has been solved. What is the structure of C2H2 on the basis of hybridization.
Which of the following is the most likely Lewis structure for C2H2. Commonly called acetylene has o 2 single bonds 1 triple bond and 1 lone pair. 70 More Lewis Dot Structures.
Thus the Lewis structure of C2H2C2H2 is. Question 7 1 pts The best Lewis structure for ethyne C2H2. I Draw the Electron dot structure ii.
I quickly take you through how to draw the Lewis Structure of CHCH Acetylene or ethyne. In the Lewis structure for acetylene the three lines between the carbon atoms represent three bonds between the carbon atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds.
Bonded to each other Answer. This is a total of 10 valence electrons that have to be included in the Lewis structure. For C 2 H 2 you have a total of 10 valence electrons to work with.
11 WRITE THE LEWIS STRUCTURE OF THE FOLLOWING COMPOUNDS 5pts each a. C2h2 would turn into what is known as Ethyne. In this tutorial we are going to learn how to draw the lewis structure of C 2 H 2 step by step.
C2h2 Lewis Structure C2H2 Lewis Dot Structure Geometry YouTube C2H2 Molecular Geometry Shape and Bond Angles see Hybridization Chapter 3. Drawing the Lewis Structure for C2H2 - Ethyne or Acetylene. Since there is only one possible lewis structure C2H2 does not have resonance.
The Lewis structure of a compound can be generated by trial and error. I also go over hybridization shape sigma pi bonding and bond ang. Draw the electron dot structure.
Other examples include CO2 HCN C2H4 C2H2 Lewis structure can. Who are the experts. H CỰC H HCc.
For molecules with resonance each lewis structure individually does not accurately depict the structure of the molecule. In drawing the Lewis structure for C 2 H 2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds. C2H2 Lewis structure Setup Step-3.
The three lines between the carbon atoms represent 8 valence electrons 2. Draw the best Lewis structure for C2H2 by filling. A H 2 S c SO 3 b CH 2 Br 2 d HCN 3 Draw the Lewis dot structure for each of the following polyatomic ions.
A molecule has resonance if more than one lewis structure can be drawn for that molecule. A NH 4 c PO 4 3 b NO 3 d CO 3 2 4 For the following molecules or ions where the central atom is underlined. To draw a Lewis Structure of any molecule and understand the bond formation in any molecule it is essential to know the total number of valence electrons.
The carbon atom has four valence electrons in its outer shell but here as there are two Carbon atoms we will multiply the. O 2 single bonds 1 double bond and 2 lone pairs. Two Hydrogens are on the top and lower parts and sandwiched in btwn these two is the chained portion of carbon-carbon and the 3rd and 4th hydrogen atom.
Now we have to determine the central atom in C2H2The central atom is that kind of atom that is single or that has lower electronegativityIn case of C2H2CarbonC is the central atom which has two atoms and H is the outer atomHydrogen is always considered as outer atom C2H2 Lewis structure Setup Step-4. There are no lone pairs on carbon or hydrogen atoms. O 2 double bonds and 2 lone pairs.
Acetylene Ethyne Lewis Structure. With C2H2 you are going to run out of valence electrons and will have to share. Download How To Draw Lewis Dot Structure For C2h2 - 2 Draw the Lewis dot structures for each of the following molecules.
C 2 H 2 acetylene or ethyne contains two carbon atoms and two hydrogen atoms. Each carbon atom has 4 valence electrons and each hydrogen has 2 valence electrons. Show transcribed image text Expert Answer.
Valence electrons in C2H2. C2h2 Dot Diagram - duflot-conseilfr symbol-ridge - symbol. Acetylene was found in whole gas octane levels 87 89 and 92 at 00022 00032 and 00037 ppbC respectively 1.

C2h2 Acetylene Ethyne Lewis Structure

Lewis Structure For C2h2 Ethyne
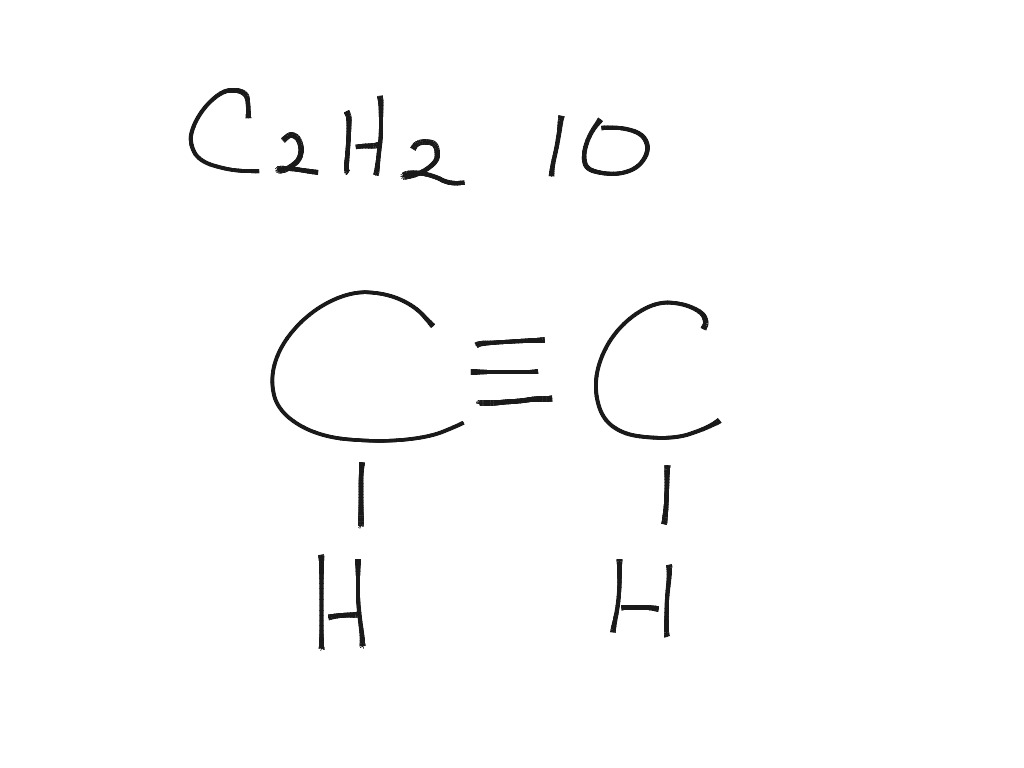
C2h2 Lewis Dot Diagram Chemistry Showme

Draw And Explain The Lewis Structure Of C2h2 Study Com

What Is The Lewis Structure Of C2h2 Study Com
Lewis Structure For C2h2 Ethyne

C2h2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist

Hcch Lewis Structure How To Draw The Lewis Structure For The Hcch Youtube

Hcch Lewis Structure How To Draw The Lewis Structure For The Hcch Youtube

Which Is The Correct Lewis Structure For Acetylene C2h2 Brainly Com
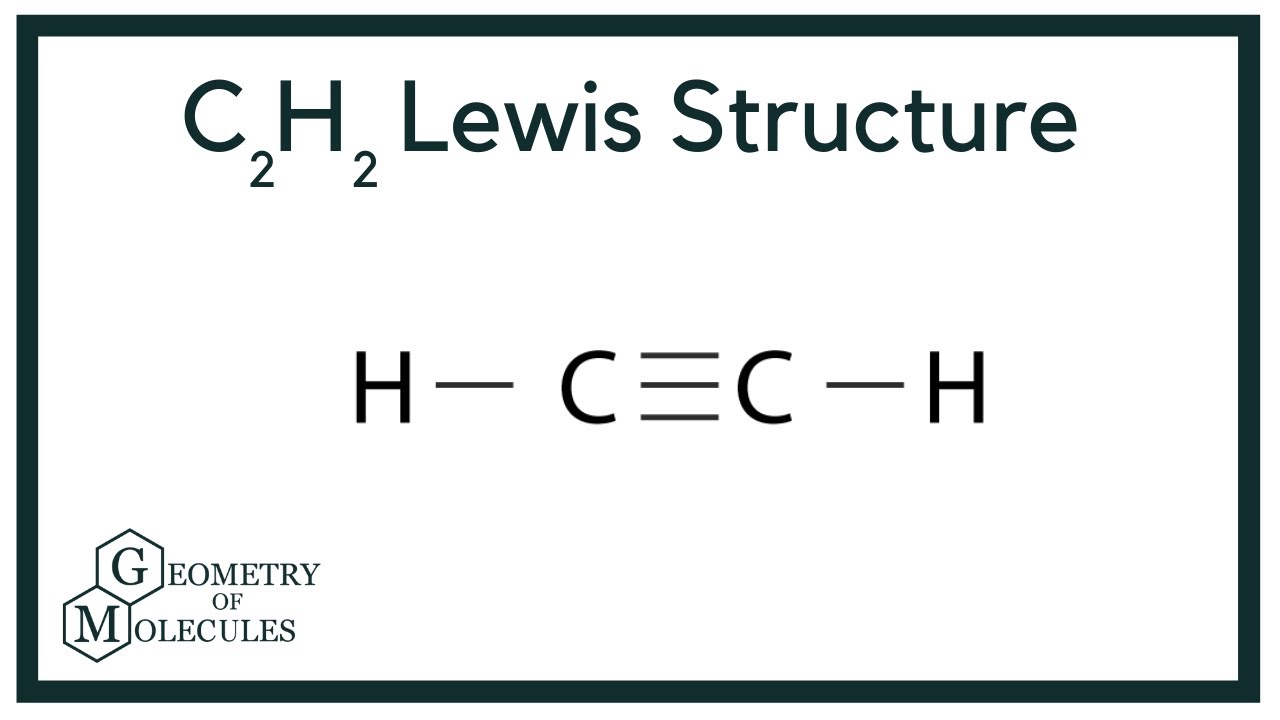
C2h2 Lewis Structure Ethyne Or Acetylene Youtube
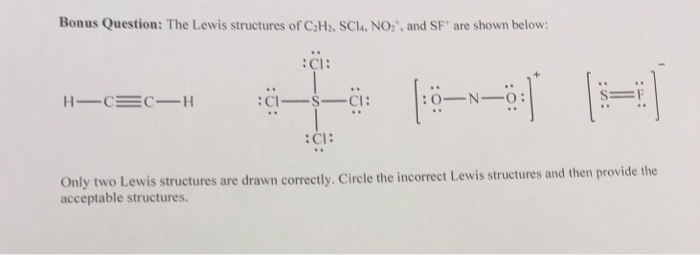
Bonus Question The Lewis Structures Of C2h2 Scla Chegg Com
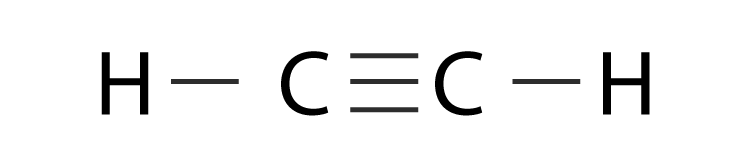
Hybridization Of C2h2 Hybridization Of C In Acetylene Ethyne

C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle
C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle

C2h2 Molecular Geometry Shape And Bond Angles See Description For Note Youtube
C2h2 Lewis Structure Tutorial How To Draw The Lewis Structure For Ethyne Or Acetylene دیدئو Dideo
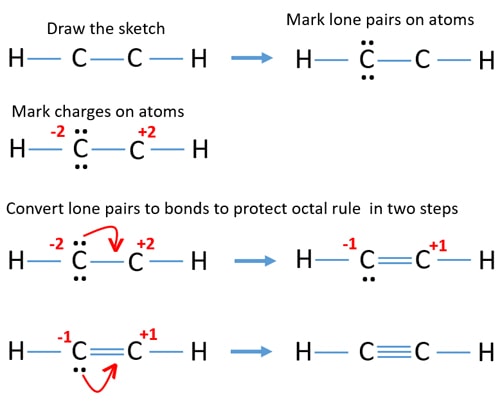
C2h2 Acetylene Ethyne Lewis Structure

C2h2 Lewis Dot Structure Geometry Youtube