Lewis Dot Structure For C2h4
What is the Lewis structure of benzene. It means four CH bond has 8 shared pairs of electrons and CC bond has 4 shared pairs of electrons.

Write Lewis Structure Of C2h4 Brainly In
In benzene itself these atoms are hydrogens.

Lewis dot structure for c2h4. Find octet e- for each atom and add them together. What is the hybridization of C in ethylene. It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule.
Its C2H4 and we want to write the dot structures for ethene. Find valence e- in all atoms. In fact Lewis structures are very important for predicting geometry polarity and reactivity of inorganic compounds.
There are two triangles overlapping each other as we can see in the diagram. What is the electron dot formula of C2H4. Note that the C2H4 Lewis dot structure involves.
Fill in the orbital diagram of unhybridized valence electrons in an isolated C atom. Lewis structures of acetaldehydeethylene oxide and vinyl alcohol. The Lewis electron dot structures of a few molecules are illustrated in this subsection.
Hutchinson 2009-09-01 The Practice of Chemistry-Donald J. Lewis dot structure of C 2 H 4. Lewis Structure of CO2.
How many shared pairs of electrons are in the lewis dot structure of C2H4. This means that the carbon atoms share 4 electrons. Each of the carbons represented by a corner is also bonded to one other atom.
Dot is used to show the valance electrons. The Lewis structure of C2 H4 also known as ethene has two carbons with a double bond between them. How_to_do_lewis_dot_structure_for_c2h4 24 How To Do Lewis Dot Structure For C2h4 Books How To Do Lewis Dot Structure For C2h4 Concept Development Studies in Chemistry-John S.
The electron dot structure is drawn using Lewis-dot structure. Two dots between two atoms represent a covalent bond. The Lewis structure of C2 H4 also known as ethene has two carbons with a double bond between them.
As per the Ethene Lewis dot structure Four CH sigma bonds are present and one CC double bond1 sigma 1 pie bond. This means that the carbon atoms share 4 electrons. Oxygen contains 6 valence electrons which form 2 lone pairs.
Electron Dot Structure for ethane C2H4. Lewis Structures for C2H4. Since it is bonded to only one carbon atom it.
Lewis Structures for C2H4. Carbon has 4 valence electrons hydrogen has 1 valence electron. In C2H4 Lewis Dot structuretwo double bonds exist between the two carbon atomsBesideseach carbon has two hydrogen atoms using two single bondsIn C2H4 Lewis Dot structurethere are totally twelve valence electrons.
Use information from step 4 and 5 to draw the lewis structure. In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair. Lets take a look.
What is the electron dot structure of cyclohexane. According to the VSEPR chart the shape of the ethene molecule is trigonal planar. How to Draw the Lewis Dot Structure for CH4.
Lewis structure also called electron-dot structure is a structural formula in which electrons are represented by dots. To do that we always count our valence electrons up first. The key to understanding how to distribute the valence electrons is to recognize the need for a double.
Carbon is in group 4 sometimes written 14 so it has 4 valence electrons. The usual structural representation for benzene is a six carbon ring represented by a hexagon which includes three double bonds. A Draw the Lewis structure for ethylene C2H4 b List the electronic and molecular geometries for C2H4 around the central C atom the C atoms are equivalent.
According to electron-dot structure there are 36 number of bonding electrons and 0 number of non-bonding electrons. If we come way over here to Hydrogen its in group 1. Hence total shared pairs of electrons in the Lewis dot structure of C2H4 is 12.
MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure MethaneFor the CH4 structure use. Watch the video of Dr. It consists of two carbon molecules and 4 hydrogen molecules.
Step-by-step tutorial for drawing the Lewis Structure for C2H4. Calculate the total valence electrons in the molecule. Welcome to Geometry of Molecules and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure.
Wink 2003-03 Students cant do chemistry if they cant do the math. Drawing the Lewis dot structure for C2H4 ethene and answer the questions below. Alternatively a dot method can be used to draw the lewis structure.
Electron dot structure of C2H4 H2CCH2. It has 1 valence electron. The central atom of this molecule is carbon.

A Compound X Is C2h4 Draw Its Electron Dot Structure Will It Dissolve In Water Brainly In

Draw The Lewis Structure For The C2h4 Ske Clutch Prep
Https Sacs Instructure Com Courses 13400 Files 607428 Download Wrap 1
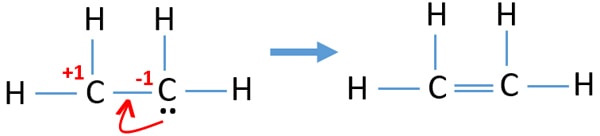
Ethene C2h4 Lewis Structure Hybridization
Lewis Electron Dot Structures Ck 12 Foundation
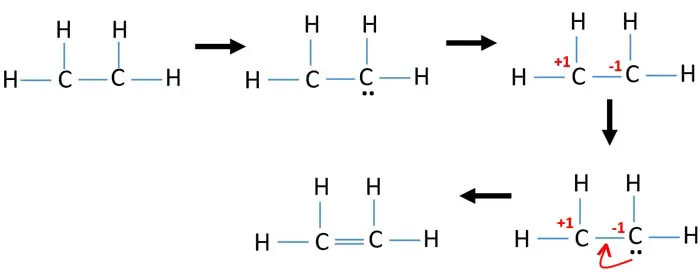
Ethene C2h4 Lewis Structure Hybridization

Draw The Electron Dot Structure Of Ethene C2h4 Brainly In
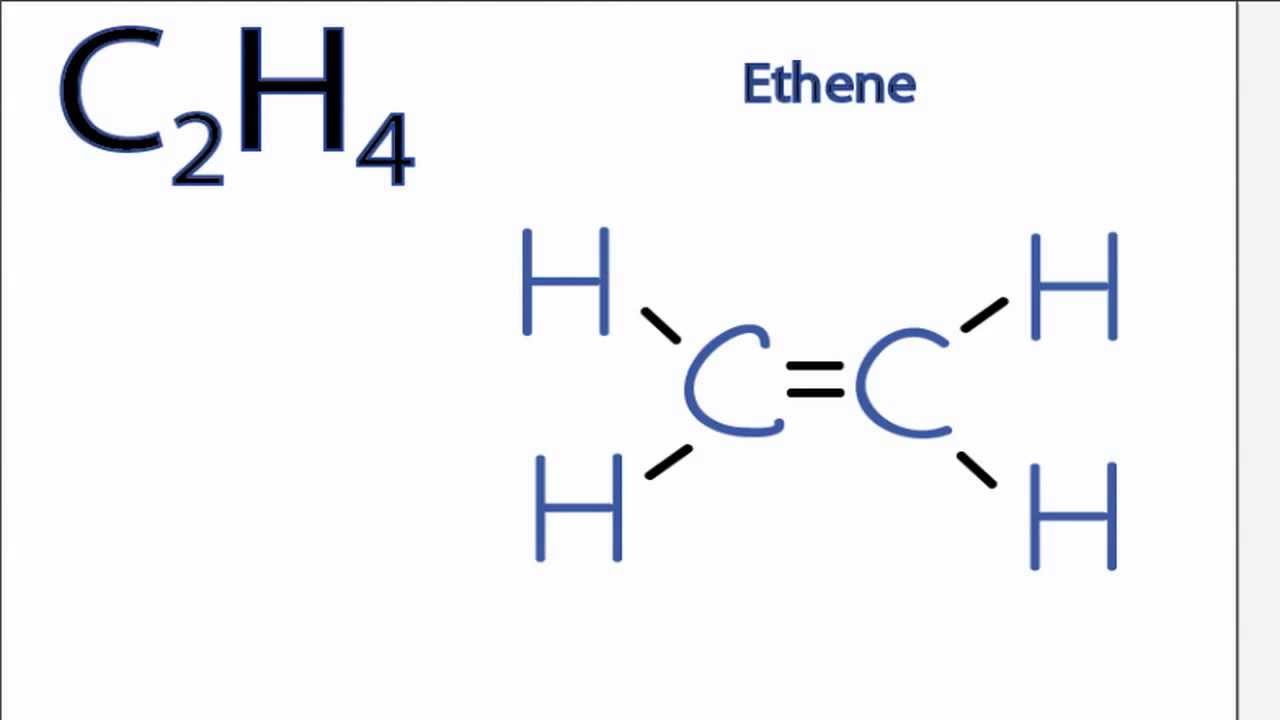
Is C2h4 Polar Or Nonpolar Youtube

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
![]()
Draw And Explain The Lewis Structure Of C2h4 Study Com

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar

Write The Electron Dot Structure Of Ethene Molecule C2h4
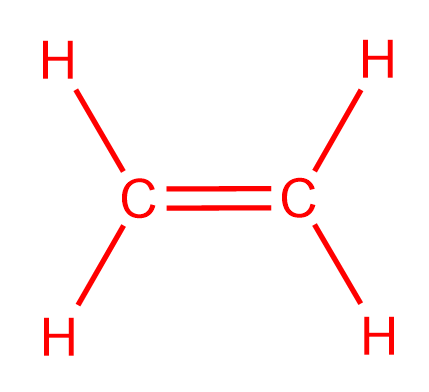
Draw The Lewis Structure For The C2h4 Ske Clutch Prep
Lewis Structure Of C2h4 Biochemhelp

C2h4 Molecular Geometry Shape And Bond Angles Youtube

C2h4 Lewis Structure C2h4 Lewis Structure Molecular Geometry

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
