Lewis Structure For Ethene C2h4
Hybridization of atoms in ethene molecue can be found from lewis structure. In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair.

Structure And Bonding In Ethene The Pi Bond Chemistry Libretexts
Alternatively a dot method can be used to draw the lewis structure.
Lewis structure for ethene c2h4. Since I only have 4 hydrogens to work with two on each carbon I assumed a double bond in the middle. Predict the HCH bond angle. The correct Lewis structure for ethene is shown below.
Lewis Structures for C2H4. D The Lewis electron-dot diagram for C2H4 is shown below in the box on the left. In a double bond two pairs of valence electrons are shared for a total of four valence electrons.
Experts are tested by Chegg as specialists in their subject area. C represents carbon atoms and H represents hydrogen atoms. Calculate the total valence electrons in the molecule.
Therefore the total number of valence electrons in Ethylene C 2 H 4. 8C 4H 12 Valence Electrons. The carbon atom consists of 6 electrons and hydrogen has 1 electron.
Electron Dot Structure for ethane C2H4. Hydrogen is the least electronegative element here. We have 12 available valence electrons.
These p-orbitals will undergo parallel overlap and form one σ σ bond with bean-shaped. This means that the carbon atoms share 4 electrons. The Lewis structure of C2 H4 also known as ethene has two carbons with a double bond between them.
It consists of two carbon molecules and 4 hydrogen molecules. The lewis structure of C2H4 is very easy to draw-Some steps need to follow for drawing the C2H4 Lewis dot structure 1. C2H4 Lewis Structure Ethylene Welcome to Geometry of Molecules and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure of C2H4 molecule.
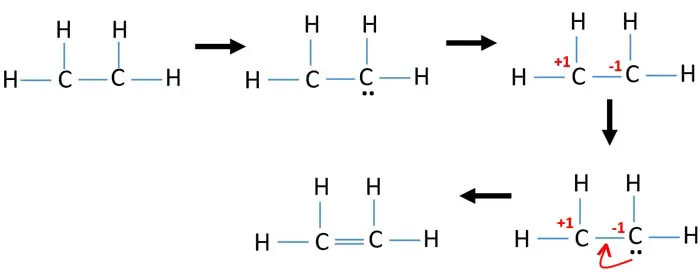
During the formation of CH 2 CH 2 the electronic configuration of carbon in its ground state 1s 2 2s 2 2p 1 2p 1 will change to an excited state and change to 1s 2 2s 1 2px 1 2py 1 2pz 1. Dot is used to show the valance electrons. The key to understanding how to distribute the valence electrons is to recognize the need for a double.
Drawing the Lewis dot structure for C2H4 ethene and answer the questions below. No lone pair is present on the central or outer atom in the lewis structure of ethene. It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule.
Be sure to include all resonance structures that satisfy the octet rule. Which is the correct lewis structure for ethylene c2h4. When we look at the molecules of C2H4 it has 2 CH molecules and 4 H molecules.
A step-by-step explanation of how to draw the C2H4 Lewis Dot Structure EtheneFor the C2H4 structure use the periodic table to find the total number of val. According to the VSEPR chart the shape of the ethene molecule is trigonal planar. Answered by ExpertThe Lewis structure for C2H4 consists of two C symbols connected by two lines.
There are two triangles overlapping each other as we can see in the diagram. Draw the Lewis structure for ethylene C2H4. However in Hydrocarbons we always place the Carbon atoms in the center as shown in the figure.
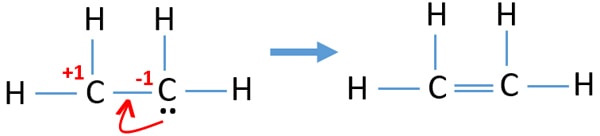
Note that the C 2 H 4 Lewis dot structure involves sharing more than one pair of electrons. Most stable structure is taken as the lewis structure of ethene. Use information from step 4 and 5 to draw the lewis structure.
Step-by-step tutorial for drawing the Lewis Structure for C2H4. In the molecule ethene both carbon atoms will be sp2 hybridized and have one unpaired electron in a non-hybridized p orbital. Lewis Dot Structure for C2H4 6 of 6 Watch the video of Dr.
Ethenes lewis structure can be built by VSEPR rule. The electron dot structure is drawn using Lewis-dot structure. Drawing the Lewis structure for C 2 H 4 named ethene requires the use of a double bond.
Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence. Ethene C 2 H 4 Lewis Structure Hybridization. Note that the entire molecule is planar.
This problem has been solved. 2 See answers SimontheSalmon SimontheSalmon Remember this when trying to find a valid lewis structure every single atom must have a total of 8 shared electrons full octet. It is a chemical formula for Ethylene or Ethene.
Ethene C 2 H 4. Count total valence electron in C2H4. Drawing the Lewis Structure for C 2 H 4.
For C 2 H 4 you have a total of 12 total valence electrons. We review their content and use your feedback to keep the quality high. C2H4 Lewis structure contains four C-H bonds and one double bond in between two carbon atoms.
Sketch the molecules shape perspective drawing. Each step of determining the lewis structure of ethene and hybridization are explained in this tutorial. Draw the Lewis structure for the ethylene C2H4 molecule.
Each C symbol is connected to two H symbols with a single line for each. In order to calculate the formal charges for C2H4 well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding ele. Lewis dot structure of C 2 H 4.
Ethene C2h4 Lewis Structure Hybridization

Hybridization Structure Of Ethylene Mcc Organic Chemistry

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Molecular Geometry Shape And Bond Angles Youtube

6 2 Lewis Structures Introductory Chemistry
Ethene C2h4 Lewis Structure Hybridization

Ethene C2h4 Lewis Structure Hybridization
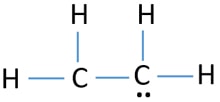
Ethene C2h4 Lewis Structure Hybridization
Lewis Structure Of C2h4 Biochemhelp
Lewis Electron Dot Structures Ck 12 Foundation
![]()
Draw And Explain The Lewis Structure Of C2h4 Study Com

Write The Electron Dot Structure Of Ethene Molecule C2h4

Hybridization In Molecules Containing Double And Triple Bonds Introduction To Chemistry

Solved A Draw Lewis Structures For Ethane C2h6 Ethylene C2h Chegg Com

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Lewis Electron Dot Structures Ck 12 Foundation

Draw The Electron Dot Structure Of Ethene C2h4 Brainly In

Draw The Lewis Structure For Ethylene C2h Clutch Prep


