Lewis Structure Of C2h4f2
Yes XeF4 or xenon tetrafluoride has a Lewis structure. 11-Difluoroethane or DFE is an organofluorine compound with the chemical formula CHF.
You may want to start by drawing all of the Lewis structures first.
Lewis structure of c2h4f2. Identify the type of intermolecular forces in each species. Carbon EN 25 is less electronegative than Bromine EN 28 and hydrogen can only make 1 bond so carbon is the central atom. It is also used as a propellant for aerosol sprays and in gas duster products.
Put two canbon atoms in the center side by sidePut one fluorine on each carbon atom. 11-Difluoroethane or DFE is an organofluorine compound with the chemical formula C2H4F2. Drawing the Lewis Structure for C 2 H 4.
Determine the central atom in this molecule. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence. Gives you bonding e-.
Indicate which of the compound s are polar. Draw three Lewis structures for compounds with the formula C 2 H 2 F 2. CH 3 CH 2 OH or CH 3 CH 2 Cl C Greatest Viscosity.
Draw the Lewis structure for the molecule. Carbon is the least electonegative atom so it goes at the center of the C 2 H 2 Br 2 Lewis structure. For C 2 H 2 you have a total of 10 valence electrons to work with.
It is also used as a propellant for aerosol sprays and in gas duster products. Calculate the total number of valence electrons present. What does each atom need to do to satisfy the octet rule like a noble gas.
CH 3 2 NH or CH 3 3 N D Largest ΔH vap. Circle the member of the pair with the corresponding property. Lewis Structures Hybridization and Geometries Fill out the table below.
For the molecule we expect the carbons to be the central atoms in this molecule since carbon tends to be the central atom in its compounds. H 3 O Lewis Structure. This colorless gas is used as a refrigerant where it is often listed as R-152a refrigerant-152a or HFC-152a hydrofluorocarbon -152a.
This may be the Lewis Structure But i am not 100 S. Do XeF4 have a Lewis structure. Calculate the total valence electrons in the molecule.
Consult with your instructor and then complete the rest of the table. Lewis structures of acetaldehydeethylene oxide and vinyl alcohol. Drawing the Lewis Structure for C 2 H 2 Ethyne or Acetylene.
In drawing the Lewis structure for C 2 H 2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds. Remember that Hydrogen H atoms always go on the outside of a Lewis Structure. In a double bond two pairs of valence electrons are shared for a total of four valence electrons.
The valency of carbon is four so it likes to have exactly four bonds so if the each carbon atom is bonded to two non - carbon atoms the. Subtract step 1 total from step 2. With C 2 H 2 Br 2 there are only single bonds.
Find the number of nonbonding lone pairs e-. Include formal charges as necessary Electronic Molecular Polar or Compound Lewis Structure Hybridization Geometry Geometry Nonpolar. Subtract step 3 number from step 1.
Know the Lewis dot structures of the highlighted atoms. Alternatively a dot method can be used to draw the lewis structure of C 2 F 2. Drawing the Lewis Structure for C 2 H 2 Br 2.
This is the Lewis Dot Structure. Even though the structures may look different when drawn 2 dimensionally on paper. Draw a dot for each valence electron around each atomic symbol.
The solution is to share three pairs of valence electrons and. Note that Hydrogen only needs two valence electrons to. Find valence e- in all atoms.
As an alternative to chlorofluorocarbons it has an ozone depletion potential of zero a lower global warming potential and a shorter atmospheric lifetime. NH 4 Lewis Structure. Drawing the Lewis structure for C 2 H 4 named ethene requires the use of a double bond.
Or if the closest noble is helium which can only have a maximum of two electrons what does it need to do to obtain a duet. Draw the Lewis dot structure for each atom in the formula. A Lowest boiling point.
Find octet e- for each atom and add them together. CCl 4 or CF 4 B Highest vapor pressure. Likewise the two structures.
Draw the Lewis Structure for each molecule. This colorless gas is used as a refrigerant where it is often listed as R-152a or HFC-152a. For Nonpolar C2H2Br2 one isomer.
13 are really the same because of free rotation found the second and third C. For C 2 H 4 you have a total of 12 total valence electrons.

Draw A Lewis Structure Of Formaldehyde Lewis Chemistry Draw

Sf4 Lewis Structure How To Draw The Lewis Structure For Sf4 Teaching Chemistry Chemistry Worksheets Chemistry Education

Ch4 Lewis Structure Methane In 2021 Lewis Methane Chemical Formula

Ch2f2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

So4 2 Lewis Structure How To Draw The Lewis Structure For So4 2 Sulfate Ion This Step By Step Teaching Chemistry Chemistry Classroom Chemistry Worksheets

Ch2f2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

126 Clo4 Lewis Structure How To Draw The Lewis Structure For Clo4 Perchlorate Ion Youtube Teaching Chemistry Chemistry Classroom Chemistry Worksheets

Http Www Youtube Com Watch V Qojoaosk5ui Lewis Chemistry Math Equations

Ch2f2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

C2h2 Lewis Structure Ethyne Or Acetylene In 2021 Math Equations Lewis Molecules

Lewis Configuration Of The Allylic Carbocation Chemistry Math Equations Lewis

126 Hso4 Lewis Structure How To Draw The Lewis Structure For The Bisulfate Ion Youtube Science Chemistry Chemistry Organic Chemistry

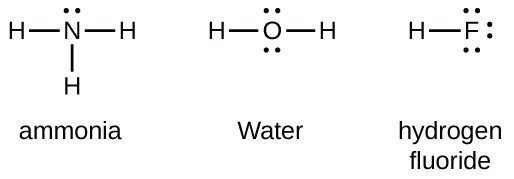
6 2 Lewis Structures Introductory Chemistry

Ch2o Lewis Structure Methanal Or Formaldehyde In 2021 Methanal Molecules Lewis

Covalent Problem C2h4f2 Isomers Youtube

6 2 Lewis Structures Introductory Chemistry

Ch2cl2 Lewis Structure Dichloromethane In 2021 Molecules Math Equations Hydrogen Atom

6 2 Lewis Structures Introductory Chemistry

6 2 Lewis Structures Introductory Chemistry
