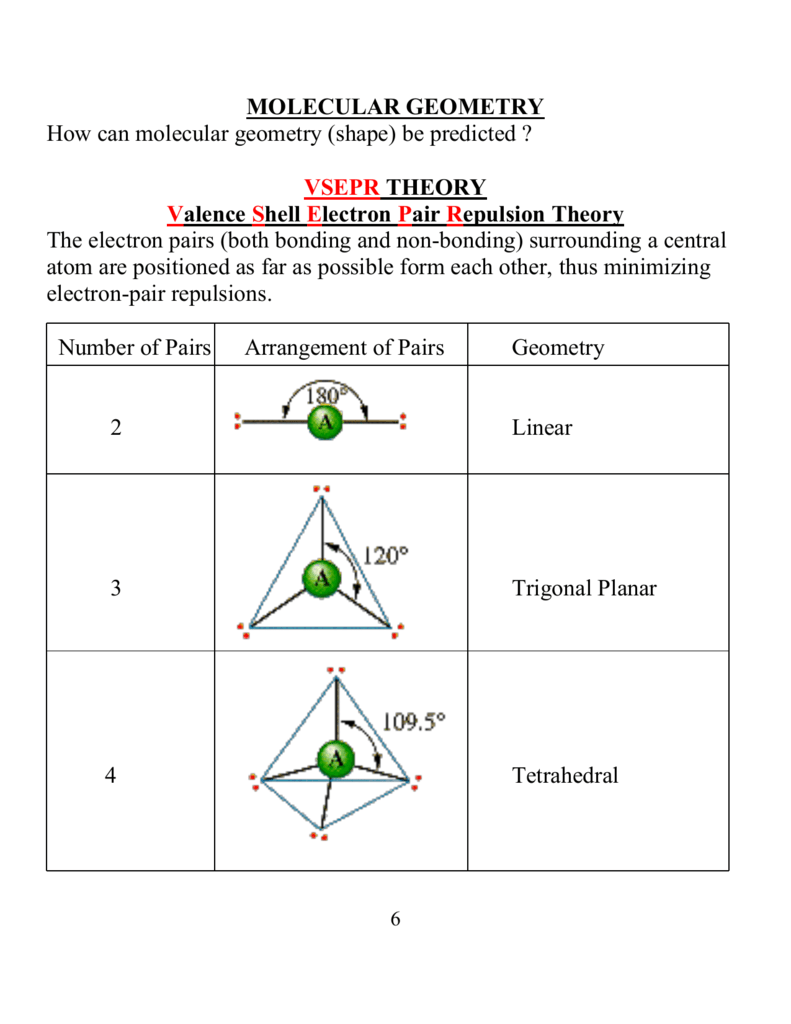
Molecular Geometry Of Cf2h2
This is the electrostatic potential of the molecule. Hydrocarbon is an organic compound which contains only carbon and hydrogen.

Electron And Molecular Geometries Molecular Geometry Teaching Chemistry Chemistry
Since the AlCl3 molecule doesnt contain any lone pair on the central atom and has a three region of electron density hence it forms a trigonal planar geometry.

Molecular geometry of cf2h2. Linear polar o Trigonal planar nonpolar Tetrahedral polar Bent nonpolar Bent ionic Trigonal pyramidal nonpolar Tetrahedral ionic Trigonal pyramidal polar Lincar nonpolar Trigonal planar ionic Trigonal pyramidaloni Den polin clone Tilastot Determine the molecular geometry and molecular polarity for periodate. Saturated hydrocarbons are those hydrocarbons which contain carbon-hydrogen and carbon-carbon single bonds. It helps with determining polarity phase of matter magnetism reactivity color and biological activity of a molecule in short anything and everything about a molecule can be studied through molecular geometry.
What is the molecular geometry of CH2F2. A quick explanation of the molecular geometry of OH- the Hydroxide io including a description of the OH- bond anglesLooking at the OH- Lewis structure we. The vibrational frequencies were calculated using the highest level of theory DZV.
Note the Hydrogen atoms H should not have lone pair. Carbon dioxide is therefore linear in electron-group geometry and in molecular geometry. The common name for this molecule is acetylene.
Intermediate colors represent intermediate potentials. Thus it has a steric number of 4. 10 What is the molecular geometry and molecular polarity of difluoromethane CF2H2.
Molar Mass Molecular Weight and Elemental Composition Calculator Enter a chemical formula to calculate its molar mass and elemental composition. A steric number of 4 with 0 lone pairs means that according to VSEPR theory CH2F2 has a tetrahedral geometry. What Is The Molecular Geometry And Molecular Polarity Of Difluoromethane CF2H2.
According to the VSEPR theory any molecule that has three regions of electron density with no lone pair on the central atom always forms a trigonal planar geometry whereas a molecule that has three regions of electron density with the presence of lone pair on the central atom always forms a trigonal pyramidal geometry. The electron geometry for the. Determine The Molecular Geometry And Molecular Polarity For Periodate.
What is the hybridization of this carbon. It has 4 atoms bonded to it. Molar mass of CF2H2 is 5202339 000094 gmol.
When carbon is bonded to four other atoms with no lone electron. The shape of CO 2 is linear because there are no lone pairs affecting the orientation of the molecule. C2H2 has a linear shape given its molecular geometry is linear and all the atoms are arranged symmetrically.
The central atom is Carbon. Lets see how to find the electron and molecular geometry of the CF4 molecule-Follow some steps to find CF4 molecular geometry 1. The frequencies would equate to peaks on a IR spectrum but one was not able to be obtained.
What is the molecular geometry and molecular polarity of difluoromethane CF2H2. Therefore the linear orientation minimizes the repulsion forces. Saturated hydrocarbons are further classified into alkane open chain of carbon atoms and cycloalkane closed chain of carbon atoms.
The red area represents the lowest electrostatic potential and blue represents the highest electrostatic potential. A quick explanation of the molecular geometry of C2H2 including a description of the C2H2 bond angles. Proiect cofinanțat din Fondul European de Dezvoltare Regională prin Programul Operațional Infrastructura Mare 2014 - 2020.
Molecular Geometry of Acetylene C2H2 Studying the molecule geometry of a molecule is a fundamental step in chemistry to analyze the behavioral properties of any molecule. Find the Number of lone pairs present on the central atom of the CF4 lewis dot structure The lone pair is non-bonding electrons that dont take part in chemical bonding. An explanation of the molecular geometry for the CF4 ion Carbon tetrafluoride including a description of the CF4 bond angles.
As per CF4 lewis structure no lone pair present on the central atom carbon. Determine The Molecular Geometry And Molecular Polarity For Periodate. C2H2 has a straight-line molecular geometry consisting of a hydrogen atom bonded to a carbon atom which is triple-bonded to a second carbon atom bonded to a second hydrogen atom.

Trigonal Pyramid Molecular Geometry Molecular Geometry Molecular Shapes Chemistry Projects

Ch2f2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Molecular Geometry Card Sort By Chemistry With Confidence Tpt Molecular Geometry Sorting Cards Molecular
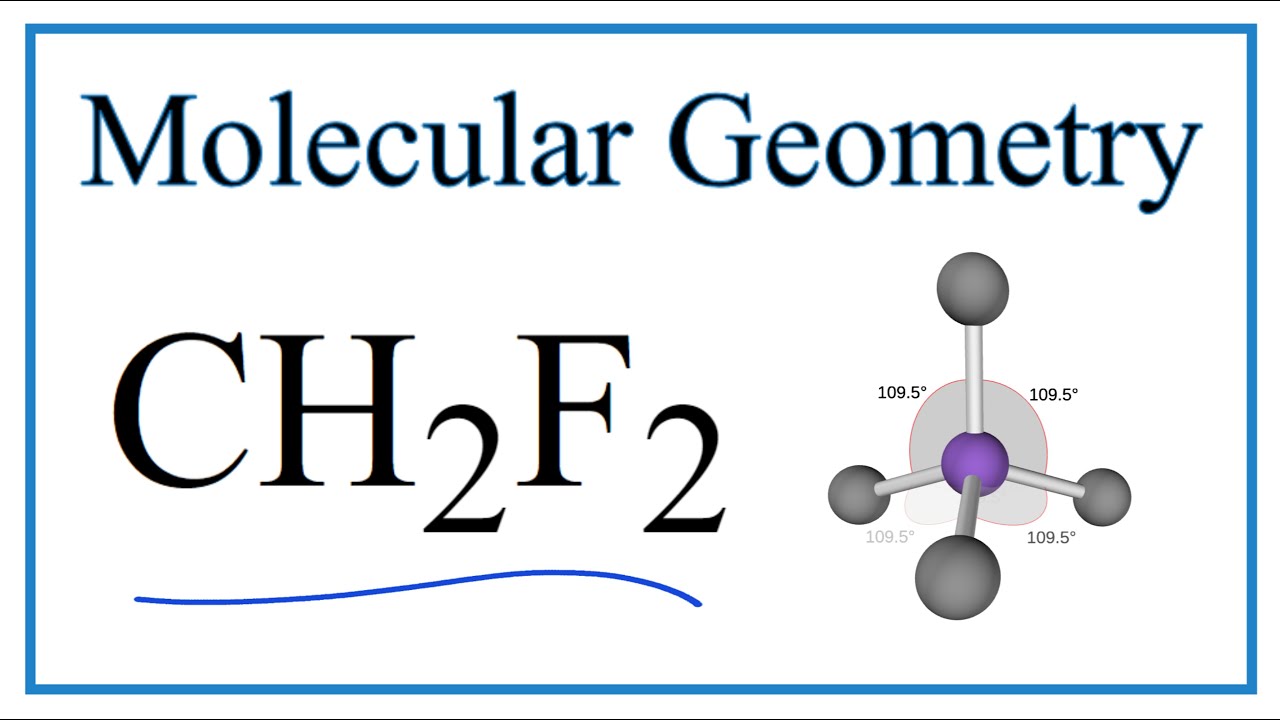
Ch2f2 Molecular Geometry Bond Angles And Electron Geometry Youtube

What Are Hybrid Orbitals Master Organic Chemistry Organic Chemistry Chemistry Molecular Geometry

Vsepr Molecular And Electron Geometry Organic Molecules Vsepr Theory Molecular Geometry
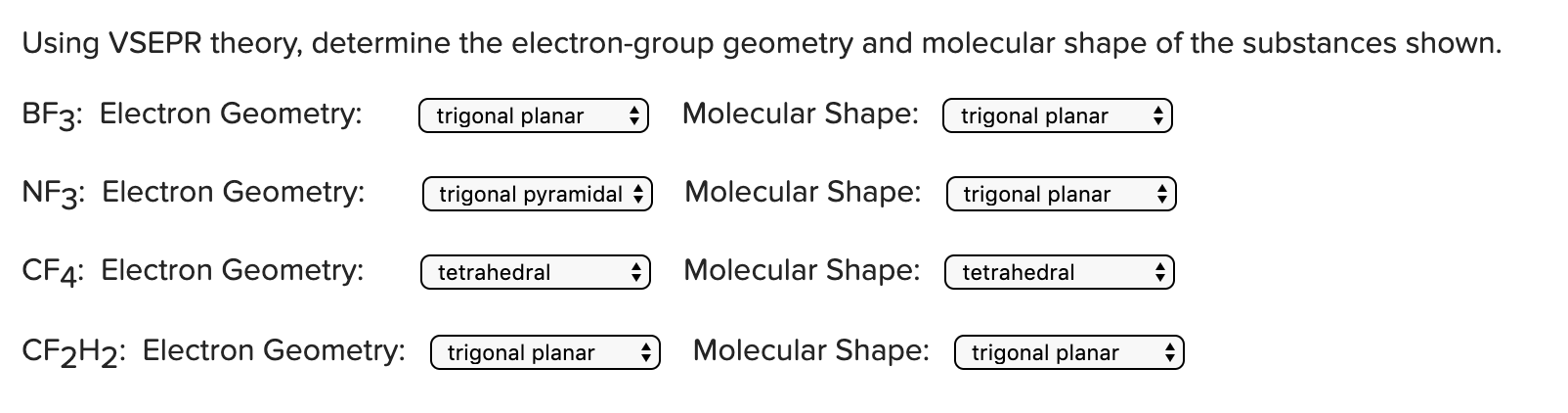
Using Vsepr Theory Determine The Electron Group Chegg Com

From Gen Chem To Org Chem Pt 7 Lewis Structures Master Organic Chemistry Organic Chemistry Molecular Geometry Organic Chemistry Books

Ch2f2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Ch2f2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Molecular Geometry Chart Molecular Geometry Molecular Geometry

Trigonal Planar Molecular Geometry Molecular Geometry Molecular Shapes Chemistry Projects

Valence Shell Electron Pair Repulsion Vsepr Chemogenesis Study Chemistry Molecular Geometry Chemistry Classroom
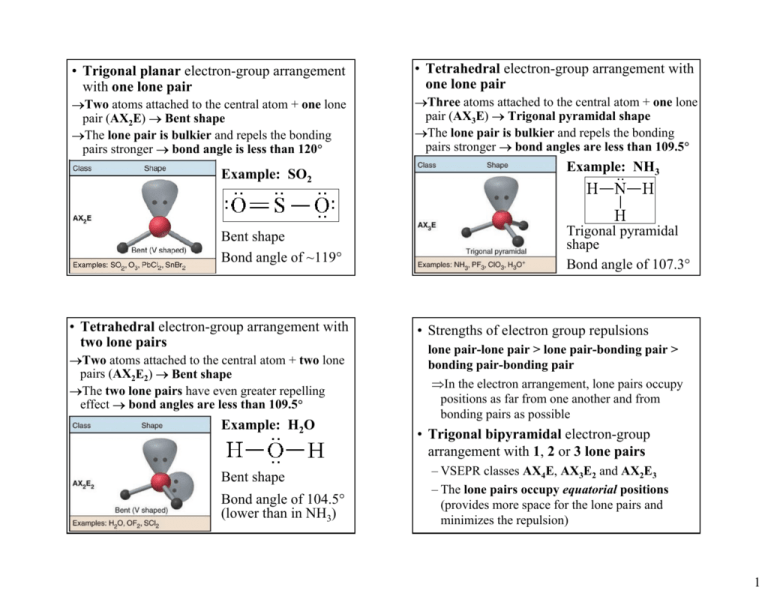
Trigonal Planar Electron Group Arrangement With One

Chf3 Trifluoromethane Molecular Geometry Bond Angles Youtube

Ch2f2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist