N2o Lewis Structure And Formal Charge
It also has a positive. Note that other two structures didnt have their atoms with the lowest possible formal charge.
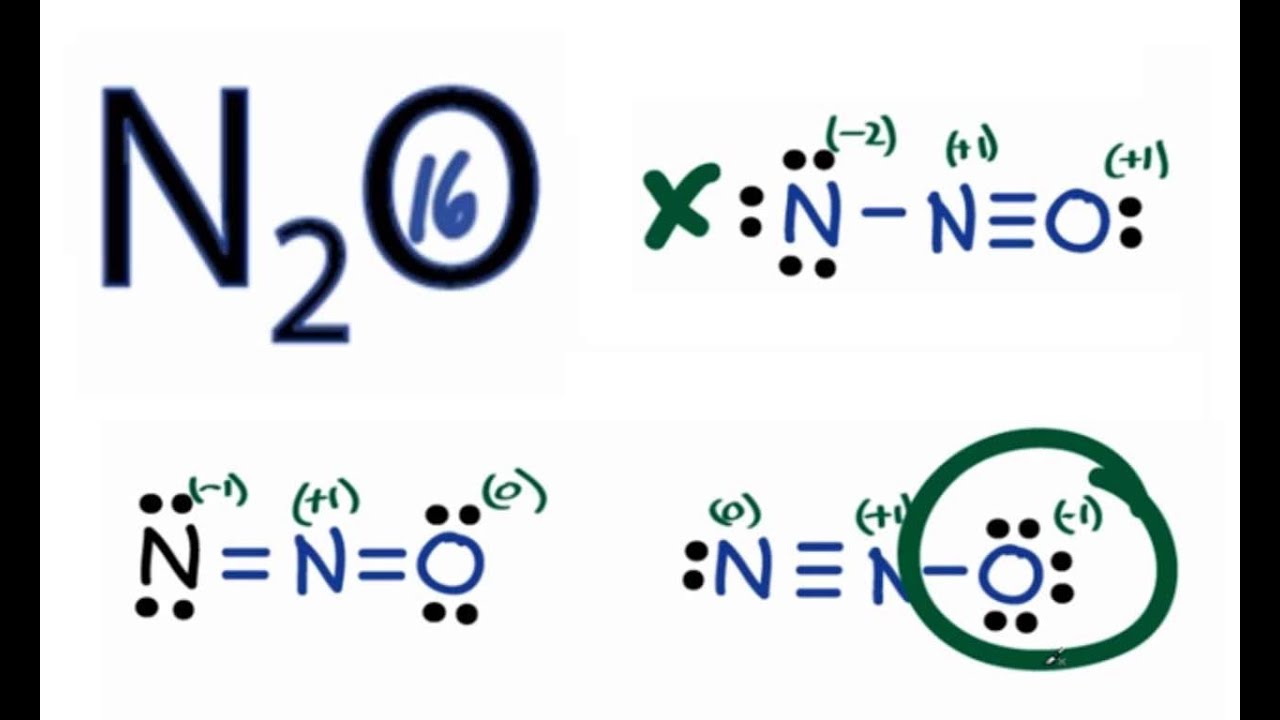
N2o Lewis Structure How To Draw The Lewis Structure For N2o Youtube
It is used in the manufacturing of semiconductors.

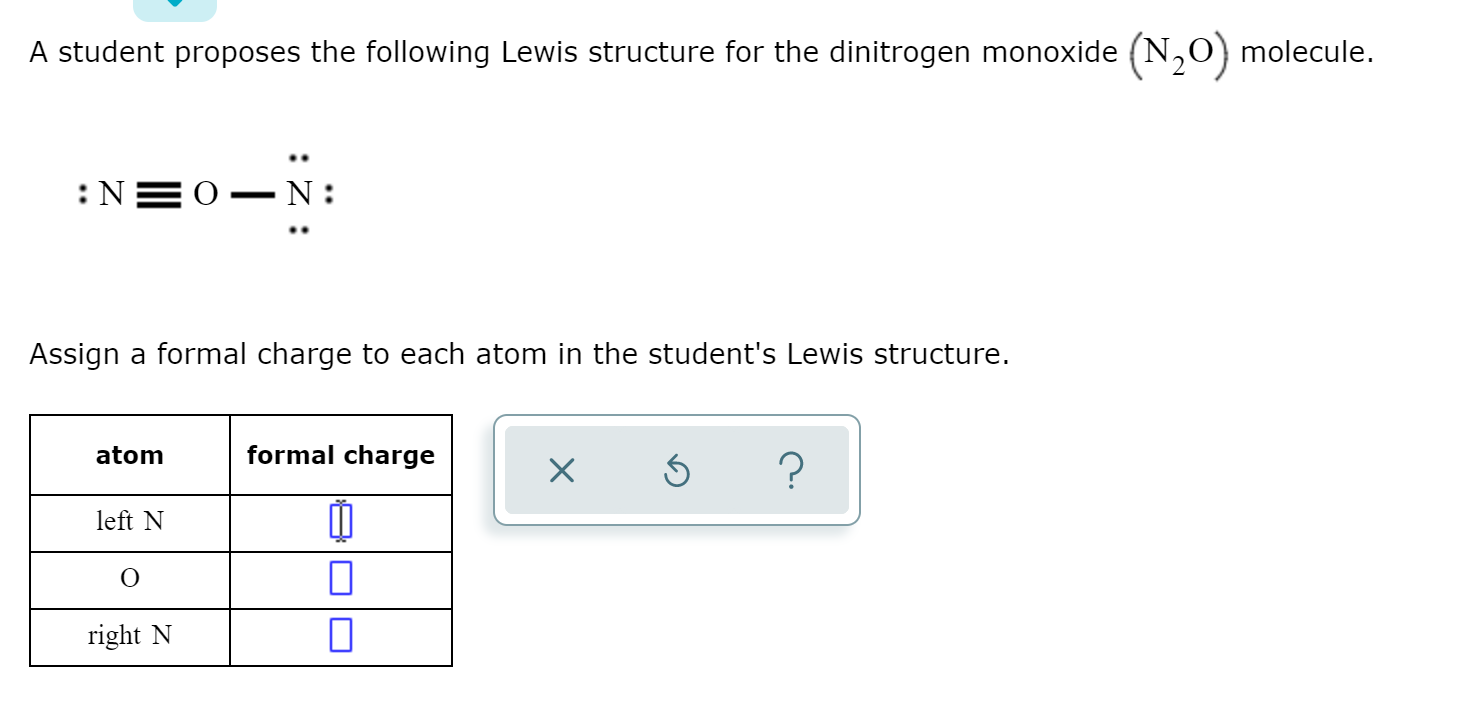
N2o lewis structure and formal charge. Since the right nitrogen owns four valence electrons one lone pair gives two electrons. A molecular structure in which all formal charges are zero is preferable to one in which some formal charges are not zero. In this case the resonance structure of 0 for N 1 for O and 1 for N has an overall formal charge of 2 while the resonance structure stated above.
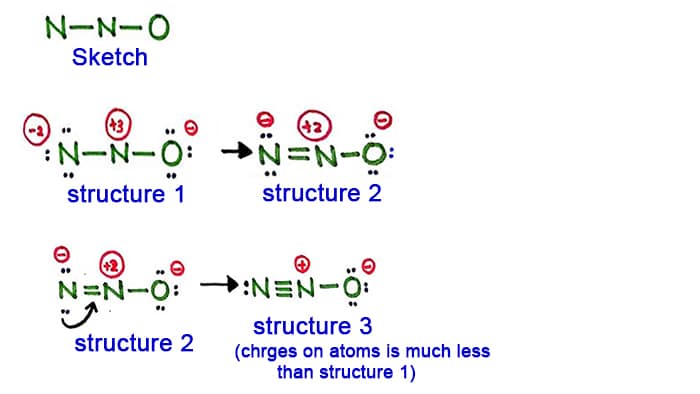
Lewis structures are preferable when adjacent formal charges are zero or of the opposite sign. Thus structure 3 is the final lewis structure of nitrous oxide. The charge of the central nitrogen atom is 1 in the N 2 O.
If the Lewis structure must have nonzero formal charges the arrangement with the smallest nonzero formal charges is preferable. The molecule SO3 has two coordinate bonds but that structure is not the most stable form as it carries a formal charge. NN0 ONEN 11 NNEO.
N2O molecule contains two nitrogen atoms and one oxygen atoms. Both formulas represent the same number of atoms and electrons just in a slightly different conformation. Formal charge on single bonded O atom 6612 2 -1.
Of non-bonding electrons-12 total no. The lowest energybest structure would have the overall formal charge minimized. As mentioned in the rules we can see all the atoms in the final lewis structure have their lowest formal charge.
The Lewis dot structures of N2O N 2 O are given below. Thus this resonance structure is not likely to occur and therefore not a structure that we would usually consider. Formal charge on atom in a molecule total no.
Now center nitrogen atom has only 1 charge and oxygen atom has a -1 charge. From the looks of this calculation the net formal charge of each structure in the molecule is higher than the overall charge of the molecule. How many bonds does N2O.
These three valid Lewis structures for dinitrogen monoxide are known as non-equivalent resonance structures. Since the left nitrogen owns five valence electrons two lone pairs and one from the NO bond and it expects five its formal charge is 5 5 0. Formal charge on double bonded O atom 6412 4 0.
Based upon formal charge which is the best Lewis structure for nitrous oxide N 2 O. Number of electrons in the valence shell of oxygen atom 6. Is dative bond present in bcl3.
This problem has been solved. A molecular structure in which all formal charges are zero is preferable to one in which some formal charges are not zero. Lewis structures are preferable when adjacent formal charges are zero or of the opposite sign.
Then two electrons from the NO double bond and it expects five its formal charge is 5 4 1. The third structure will be the least stable as there is greater charge separation with respect to structures I and II. N 2 O molecule contains two nitrogen atoms and one oxygen atoms.
If the Lewis structure must have nonzero formal charges the arrangement with the smallest nonzero formal charges is preferable. First we should try to draw the most stable lewis structure of N 2 O to decide the shape of N 2 O. What is the correct Lewis structure for N2O including the formal charges if any.
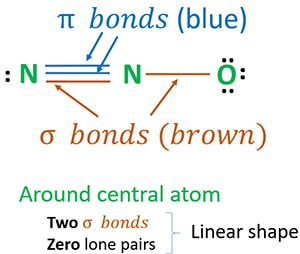
Both 1 and 2 are good structures. The most stable resonance nitrous oxide lewis structure would have the formal charges of 1 for N -1 for O and 0 for N. Its center atom contains around it two sigma σ bonds.
Number of electrons in the valence shell of nitrogen atom 5. Since the left nitrogen owns five valence electrons two lone pairs and one from the NO bond and it expects five its formal charge is 550. Most often Lewis structures are drawn so that the the formal charge of each atom is minimized.
Structure 3 is the best most stable structure we can draw for N 2 O. The Terminal Nitrogen Charge is 2- while the Nitrogen bonded to it is only 1. IV - O II O III IV OV.
Of valence electrons in the free atom- total no. Note that a Lewis structure for carbon dioxide can be written using a carbon-oxygen single bond on one side and carbon-oxygen triple bond on the other. Of bonding electrons Lewis structure of N O 2 is.
Lowest Energy Lewis Structure. Also to know is what is the structure of n2o. Resonance structures Two of the contributing structures of nitrogen dioxide NO2.
Now lets move to the molecular geometry of nitrous oxide. Shape of N 2 O molecule around center atom. As per N 2 O Lewis structure molecular geometry of N 2 O is linear.
As an assessment tool formal charge assignments can be used to predict the relative contributions of the resonance forms to the resonance hybrid which represents a more realistic conception of the electron distribution within the molecule. In order to calculate the formal charges for N2O well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding elec.

N2o Lewis Structure Nitrous Oxide Youtube

N2o Lewis Structure Nitrous Oxide Laughing Gas What S Insight
N2o Lewis Structure Resonance Structures Oxidation Number
N2o Lewis Structure Resonance Structures Oxidation Number
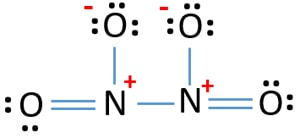
Lewis Structure Of N2o4 Dinitrogen Tetroxide Drawing Steps

N2o5 Lewis Structure How To Draw The Lewis Structure For N2o5 Youtube

N2o Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
A Student Proposes The Following Lewis Structure For Chegg Com
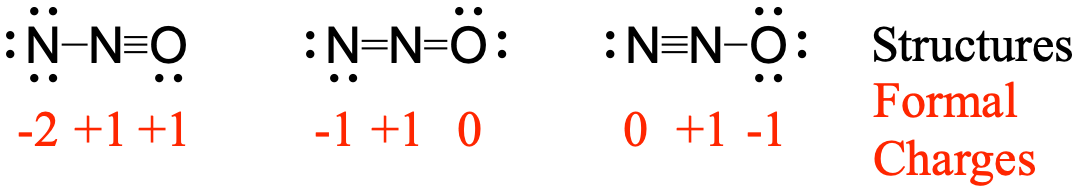
Resonance Structures And Formal Charge M8q3 Uw Madison Chemistry 103 104 Resource Book

N2o4 Dinitrogen Tetroxide Resonance Structures

N2o Lewis Structure How Can N Form A Double Bond Chemistry Community
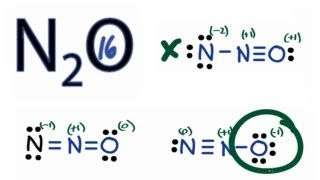
N2o Lewis Structure How To Draw The Lewis Structure For N2o Youtube
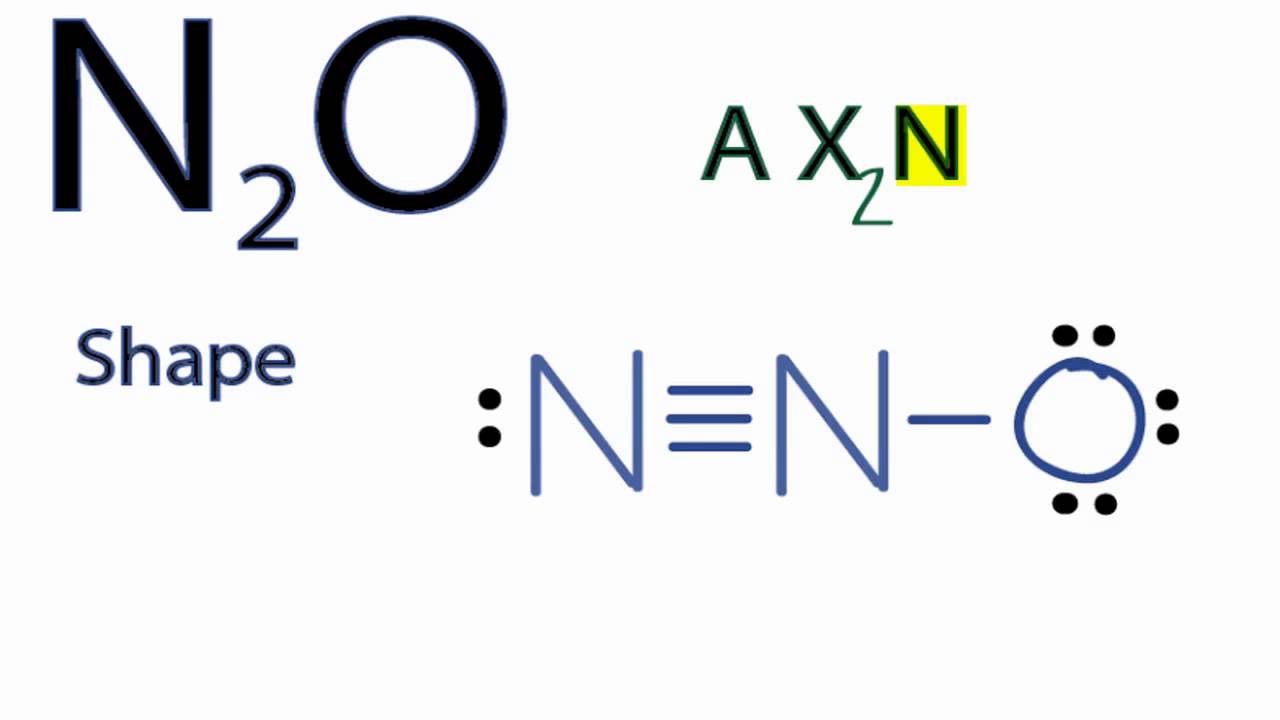
N2o Lewis Structure How To Draw The Lewis Structure For N2o Youtube

Identify The Lowest Energy Lewis Structure For Nitrogen Oxide Brainly Com

N2o Lewis Structure Nitrous Oxide Youtube

N2o Lewis Structure Nitrous Oxide Laughing Gas What S Insight

N2o Lewis Structure Nitrous Oxide Laughing Gas What S Insight