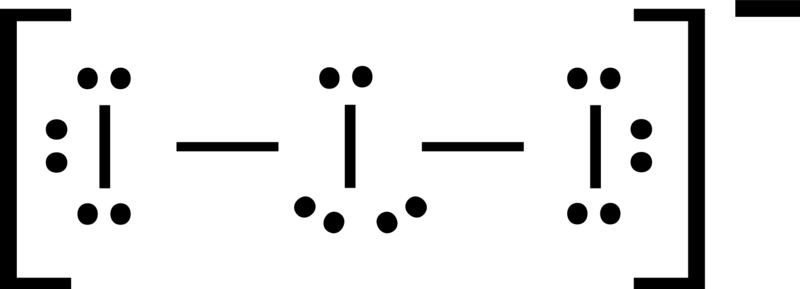Nitrogen Triiodide Molecular Geometry
They are of course polar. There are non radioactive and radioactive forms of iodine.

Using The Vsepr Model Identify The Molecular Chegg Com
The S orbital pairs of electrons around a central atom electrons around a certain atom each.
Nitrogen triiodide molecular geometry. An explanation of the molecular geometry for the NI3 ion Nitrogen triiodide including a description of the NI3 bond angles. The polarity of a molecule is directly proportional to the difference between the electronegativity of atoms involved in that molecule. Nitrogen triiodide is a covalent compound with a central nitrogen atom.
6 rows Molecular Formula. 1 tetrahedral 2 square planar 3 trigonal pyramidal 4 octahedral 5 trigonal planar N I I I 3 45. And the entire molecule ensures non zero dipole moment.
Chemistry QA Library Determine the molecular geometry and molecular polarity for nitrogen triiodide Determine the molecular geometry and molecular polarity for nitrogen triiodide close. The molecules themselves have a trigonal triangular pyramid shape. See full answer below.
NI3 is a polar molecule one must remember that the lone pairswill skew the electron geometry. Molecular geometry of NO3. The chemical formula NI 3 is named nitrogen triiodide.
The Lewis structure reveals that the central atom is nitrogen and that it is surrounded by four electron pairs which corresponds to a tetrahedral electron-pair geometry. 3 bonding pairs 0 lone pairsSteric number 4. Bbr3 BORON TRIBROMIDE is Nonpolar.
NF 3 has a small dipole moment 0234D in comparison with NH 3 142D. Determine the molecular geometry and molecular polarity for nitrogen triiodide. The Lewis structure reveals that the central atom is nitrogen and that it is surrounded by four electron pairs which corresponds to a tetrahedral electron-pair geometry.
Is NI3 polar or non polar. A step-by-step explanation of how to draw the NI3 Lewis Dot Structure Nitrogen TriiodideFor the NI3 structure use the periodic table to find the total num. 1 sp 2 sp 2 3 sp 3 4 sp 4 5 sp 3 3 Ch 102 Orbital hybridization d 46.
What is the hybridization on N in nitrogen trioiodide. Linear trigonal planar bent tetrahedral trigonal pyramidal trigonal bipyramidal seesaw T-shaped octahedral square pyramidal square planar. The Lewis structure reveals that the central atom is nitrogen and that it is surrounded by four electron pairs which corresponds to a tetrahedral electron-pair geometry.
Iodine is also added to some table salt to ensure that all people in the United States have enough. NI3 Nitrogen triiodide is Polar Ill tell you the polar or nonpolar list below. What is the molecular geometry of nitrogen triiodide.
Which do you expect to have the longest bond length. That produces minor negative charge on each chlorine in the compound and minor positive on the boron. Iodine is used as a disinfectant for cleaning surfaces and storage containers and is used in skin soaps and bandages and for purifying water.
O Linear ionic O Trigonal pyramidal nonpolar Trigonal pyramidal polar Tetrahedral ionic Bent polar Trigonal planar ionic Tetrahedral polar Trigonal planar polar Bent nonpolar Linear nonpolar Trigonal planar nonpolar Tetrahedral nonpolar Linear polar beton Determine the. An explanation for this is that the moment due to the nitrogen atom and its lone pair is in opposition to the moment associated with the three polar N-F bonds in NF 3. The Lewis structure for nitrogen triiodide shows that the central nitrogen atom has three bonding electrons and one lone pair.
For NI3 nitrogen Triiodide two axial atoms located on opposite ends of electron. Nitrogen N on its own contains 5 valence electrons as a main group 5A. Using the VSEPR model identify the molecular geometry of nitrogen triiodide NI3 based on the number of electron domains.
Nitrogen triiodide is most polar because the molecule is asymmetric while carbon disulfide is symmetric canceling out the dipoles of the bonds. Iodine is a naturally occurring element found in sea water and in certain rocks and sediments. Leaving 10 valance electrons only 8 valance electrons NI3 nitrogen Triiodide 4A molecular shape.
The Lewis structure for nitrogen triiodide shows that the central nitrogen atom has three bonding electrons and one lone pair. The electron geometry for the N.

For The Following Molecules Or Polyatomic Ions Draw Chegg Com

Ni3 Lewis Structure How To Discuss

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube
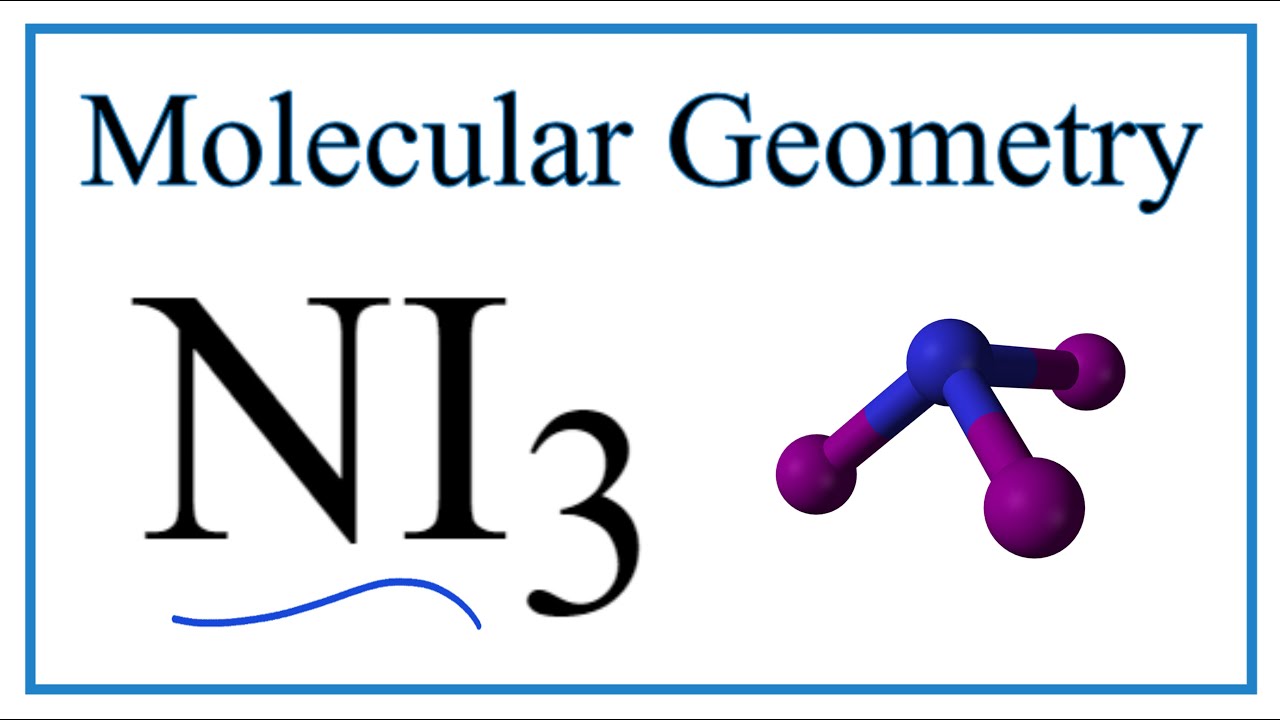
Ni3 Molecular Geometry Bond Angles Electron Geometry Youtube

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube
Chapter 3 Molecular Shape And Structure Flip Ebook Pages 1 11 Anyflip Anyflip

Ni3 Molecular Geometry Bond Angles Electron Geometry Youtube

Molecular Models Activity Carbon Tetrachloride Ammonia Methane Hydrogen

What Is The Lewis Structure Of Ni3 Study Com

Molecular Models Activity Carbon Tetrachloride Ammonia Methane Hydrogen
Molecular Geometry Ck 12 Foundation

Nitrogen Triiodide Formula I3n Over 100 Million Chemical Compounds Mol Instincts
Nitrogen Triiodide Ni3 Pubchem
Nitrogen Tri Iodide Molecule Of The Month December 2001 Jsmol Version