Pcl3 Lewis Structure Electron Geometry
This step by step chemistry video will show you how to draw Lewis structures and determine molecular geometry using phosphorus pentachloride PCl5 as an exa. To draw the lewis structure first of all we need to sum up the valence electrons of all the atoms.

Pcl3 Molecular Geometry Shape And Bond Angles Youtube
When we examine the Lewis structure of PCL3 we can see that each chlorine atoms have 3 lone pairs and all of them must have 8 electrons around it.

Pcl3 lewis structure electron geometry. Phosphorus trichloride P Cl3 P C l 3 has a central phosphorus atom with a single bond to each of the three chlorine atoms. These chlorines want to satisfy their oxide requirement and that is why the geometry for PCL3 is called Trigonal Pyramidal. The Lewis structure of a compound does not deal with the 3-dimensional representation of its elements in space nor its molecular design and geometry.
The lewis structure of PCl3 can be explained as follows. Now lets move on to the lewis structure of PCl3. Name of Name of Valence electrons Lewis Structure electron geometry molecular geometry a.
Electron pair geometry of the pcl3. Now the central atom is generally the least electronegative atom or atom with the. According to the VSEPR theory the molecular geometry of NCl3 is trigonal pyramidal and electron geometry is tetrahedral because nitrogen being pentavalent has Sp³ hybridization with 5 valence electrons in its outermost shell and it makes three bond pairs one with each chlorine atom.
There are a total of 26 valence electrons for PBr3. PCl3 Electron Geometry When you look at the Lewis Structure of the molecule you can see that electrons arrangement is in a tetrahedral geometry. Related to this Question.
The molecule is trigonal pyramidal-shaped and is a polar molecule. It has sp3 Hybridization and the bond angle is approximately 1095. Drawing the Lewis structure of PCl5.
Find the total number of valence electrons in a molecule- Adding up the valence electrons of all the atoms in a molecule is the first step. Since phosphorus has 5 valence electrons the two electrons not shared in. Well as the Lewis electron dot structure and the name of the molecular geometry shape of the following molecules.
The properly way to determine the Lewis structure based on this example is. Then learn how to predict the shape of a molecule by applying the VSEPR theory to the Lewis dot structure. But because one of those four hybridized orbitals is a lone pair there isnt really anything there just space where the electron probably is.
Now lets see the proper steps to draw a lewis structure-1. The geometry of the CH3I molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory VSEPR Theory which states that molecules will choose the CH3I geometrical shape in which the. 7cdot2 6cdot2 26 Total electrons needed for octetsdoublets.
Chemistry learning made easyThis tutorial will help you deal with the lewis structure and moleculargeometry for boron triiodide BI3. In the Lewis structure of PBr3 there are three bonding pairs of electrons and one lone pair of electrons on the central atom. Hence the electron geometry of Phosphorus Trichloride is tetrahedral.
The CH3I Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the CH3I molecule. Choose a central atom and draw a skeletal structure- Sketch a skeletal of the molecule with only single bonds. 8cdot4 32 Total sharedbonding electrons.
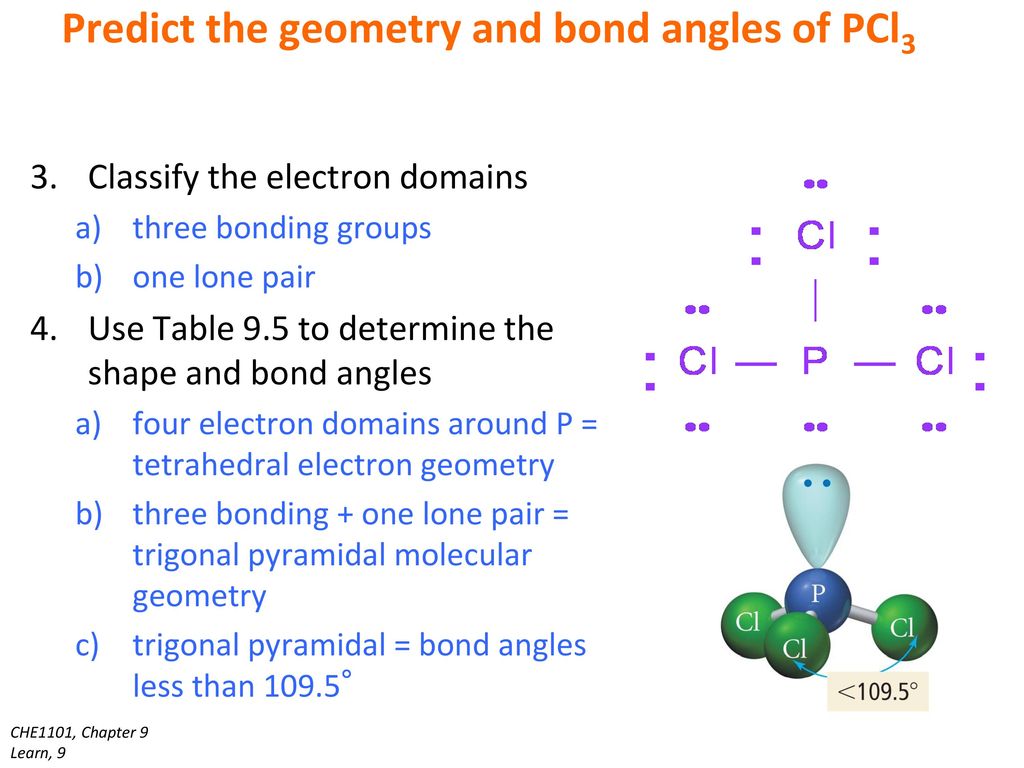
32-266 In â In the Lewis structure for PCl 3 there are a total of 26 valence electrons. Here Phosphorous 5 valence electrons Chlorine 7 valence electrons 3 Cl 73 21 So total valence electrons 26. The molecular geometry of PCl3 is trigonal pyramidal and its VSEPR notation is AX3E.
The A represents the central atom the phosphorus each X represents a chlorine atom and the E represents the lone pair. Count the number of valence electrons in a PCl5.
Your Turn A Central Atom Has Two Lone Pair Of Electrons Around It And Two Single Bonds To Other Atoms What Is The Electron Pair Geometry Around The Central Ppt Download

What Is The Molecular Shape Of Pcl3 Quora

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

How Many Lone Pair Electrons Are There In Pcl3 Quora

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3

How Can The Molecular Geometry Of Phosphorus Trichloride Be Described Quora

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

What Is The Molecular Geometry Of Pcl3 Study Com

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3

Molecular Geometry Vsepr Theory Lessons Blendspace

Pcl3 Lewis Structure And Molecular Geometry Youtube

Dublin Schools Lesson Molecular Geometry What Shapes Do Molecules Have

How To Draw The Lewis Structure Of Pcl3 Phosphorus Trichloride Youtube
Https Www Mctcteach Org Chemistry C1020 C1020 Handouts Molecular 20modeling 20v 8 18 Pdf
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization
What Is The Bond Angle Of Pcl3 Quora