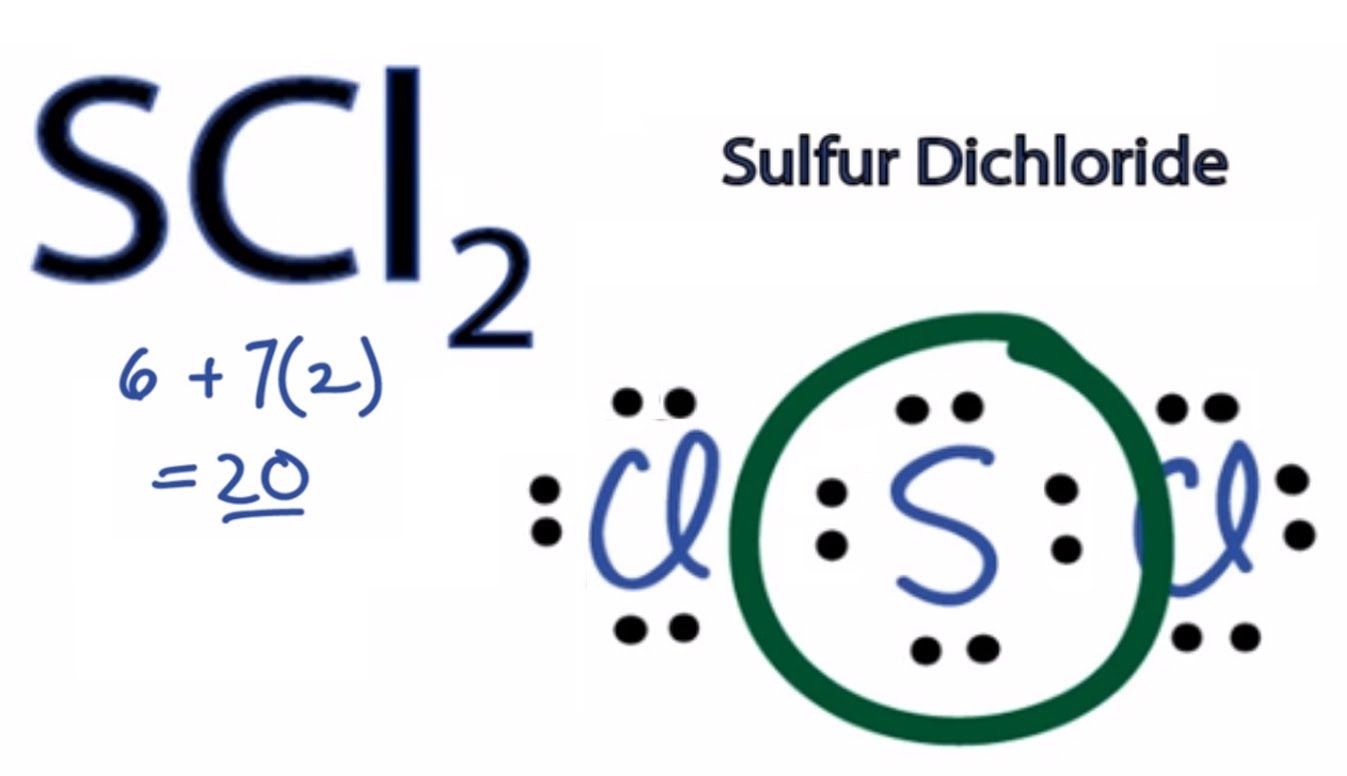
Scl2 Lewis Structure Polar Or Nonpolar
Two of the electrons are shared with two chlorine atoms to. In the Lewis structure of CH3I the formal charge on the terminal hydrogen atom is zero.

Is Cs2 Polar Or Nonpolar Youtube
Otherwise it is polar.

Scl2 lewis structure polar or nonpolar. If you want a greater understanding of the shape search Google for SCl2 Lewis Structure. The reason why SCL2 is polar its because of the bent molecule and mild difference of electronegativity between sulfur and chlorine. For TeCl_4 we have 4 bonded species about the central and 1 lone pair of electron about the central atom.
Sulfur dichloride SCl2 is a polar molecule. Chemistry QA Library Draw the Lewis electron dot structure for SCl2 and discuss its molecular geometry. The core atom is sulfur which is flanked by two chlorine atoms.
It is used as a propellent of rockets and fluorinating chemicals. Find the net dipole moment you dont have to actually do calculations if you can visualize it If the net dipole moment is zero it is non-polar. Net dipole moment and electronegativity are also responsible for the SCl2 polarity nature.
Use symmetry to determine if the molecule is polar or non-polar. With that its also essential to know about the C2H4 NF3 and SCl2 polarity. I hope its now clear to you that sulfur dichloride SCl2 is a polar molecule.
Therefore SCN is polar. Learn to determine if Cl2 is polar or nonpolar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Structure and then use. The molecular shape of Cl2 is linear.
CH 2 O f. The hybridization of each chlorine atom in Cl2 is Sp³. Is SCl2 molecule polar.
The total valence electron available for drawing the Cl2 lewis structure is 14. It contains two pairs of electrons and two polar bonds which confer a net dipole moment on the molecule due to their geometric arrangement. Chlorine atom is required one electron to complete the octet of chlorine atoms.
N 2 O i. Is SCl2 molecule polar or nonpolar. Second place the valence electron on the iodine and hydrogen atoms.
First the valence electrons are placed around the carbon atom. Draw the Lewis structure Step 2. This means it is not a linear molecule because of lone pairs of electrons which have greater repulsion and therefore the charges do not cancel out meaning the molecule is polar.
The Lewis structure for sulfur dichloride should show that two lone pairs of electrons are present on the sulfur atom. Draw the 3D molecular structure w VSEPR rules Step 3. Is SCl2 polar or nonpolar.
SCl2 is polar because of its asymmetrical shape and SCl2 has a bent molecular structure because of the lone pair present on the sulfur central atom which causes non-uniform distribution and makes its structure bent or V-shaped. CF 2 H 2 e. Sulfur has six valence electrons.
H 2 O m. The sulfur dichloride SCl2 has bent molecular geometry. SCl2 Sulfur dichloride is polar in nature because of bent geometrical shape due to the presence of lone pair present on the sulfur atom.
Figure out the geometry using VSEPR theory Visualize or draw the geometry. Steps to Identify Polar Molecules. That was all about is SCl2 polar or nonpolar.
The SCN is a polar molecule because in SCN the nitrogen and sulfur atoms attract the electrons from the carbon atom which results in a negative charge on nitrogen and sulfur and a partial positive charge on the carbon atom. SCl2 has a bent structure. Cl2 is non-polar in nature because of no dipole moment present in it.
While drawing its Lewis structure follow the above instructions or watch the video. For SCl2 provide a Lewis structure predicted VSEPR molecular geometry bond angle and indicate whether the compound is polar non polar or a polyatomic ion. CCl 2 F 2 d.
Sulfur contains six outermost valence electrons which means it contains six electrons in its outermost shell whereas Chlorine has seven outermost electrons. Bromine pentafluoride has the chemical formula BrF5 and is a pale yellow liquid. IO3 -1 iodine Is Center.
In this post we discussed the method to construct the CH3I Lewis structure. Lewis Structures Shapes and Polarity W 319 Everett Community College Student Support Services Program Draw Lewis structures name shapes and indicate polar or non-polar for the following molecules. For The Following Compounds Give Its Lewis Structure Shape And Whether Its Polar Or Non Polar1.
As a fluorinating reagent it is an interhalogen chemical with bromine and fluorine. Bromine pentafluoride BrF5 lewis dot structure molecular geometry polar or non-polar bond angle. These lone pairs of electrons are responsible for giving the molecule a bent molecular geometry much like the two lone pairs of electrons present on the oxygen atom are responsible for giving the water molecule a bent geometry.
The formal charge of Chlorine in the Cl2 lewis dot structure is zero. Secondly the difference between the electronegativity of sulfur and chlorine atoms makes the S-Cl bonds polar and as a result the entire molecule also becomes polar and gives a net dipole moment of 054D. Draw the Lewis structure.

Is Nh2 Polar Or Non Polar Amide Ion In 2021 Nh 2 Molecules Electrons
Is Scl2 Polar Or Non Polar Quora

Makethebrainhappy Is Scl2 Polar Or Nonpolar
Polar And Nonpolar Molecules Is It Polar Or Nonpolar Youtube

Is Scl2 Polar Or Non Polar Sulfur Dichloride Youtube
Is Scl2 Polar Or Non Polar Quora

Is Scl2 Polar Or Nonpolar All About Scl2 Polarity

Is Scl2 Polar Or Nonpolar All About Scl2 Polarity

Is Scl2 Polar Or Nonpolar Techiescientist

Is Scl2 Polar Or Nonpolar Techiescientist

Is Scl2 Polar Or Nonpolar Techiescientist

Why Is Scl2 Polar I Have The Lewis Structure And From There I Can See That It Has Polar Bonds Because Of The Cl So Both S Cl Bonds Are Polar Bonds

Is Scl2 Polar Or Nonpolar Techiescientist

Is Cbr4 Polar Or Non Polar Carbon Tetrabromide Youtube

Scl2 Lewis Structure Molecular Geometry Or Shape Polarity Hybridization
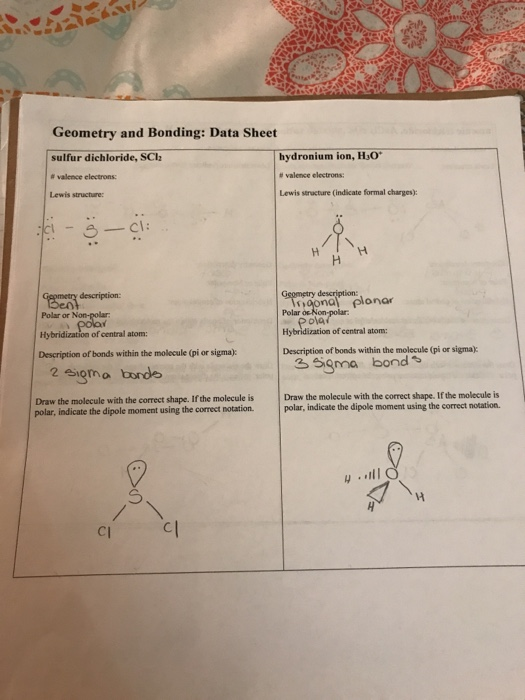
Geometry And Bonding Data Sheet Sulfur Dichloride Chegg Com

Is Xef4 Polar Or Non Polar Xenon Tetrafluoride Youtube
Is Scl2 Polar Or Non Polar Quora