Sef4 Molecular Structure
Molar mass Cu 6355g mol. This electron arrangement is known as Trigonal Bipyramidal.

Sf4 Molecular Geometry Shape Youtube
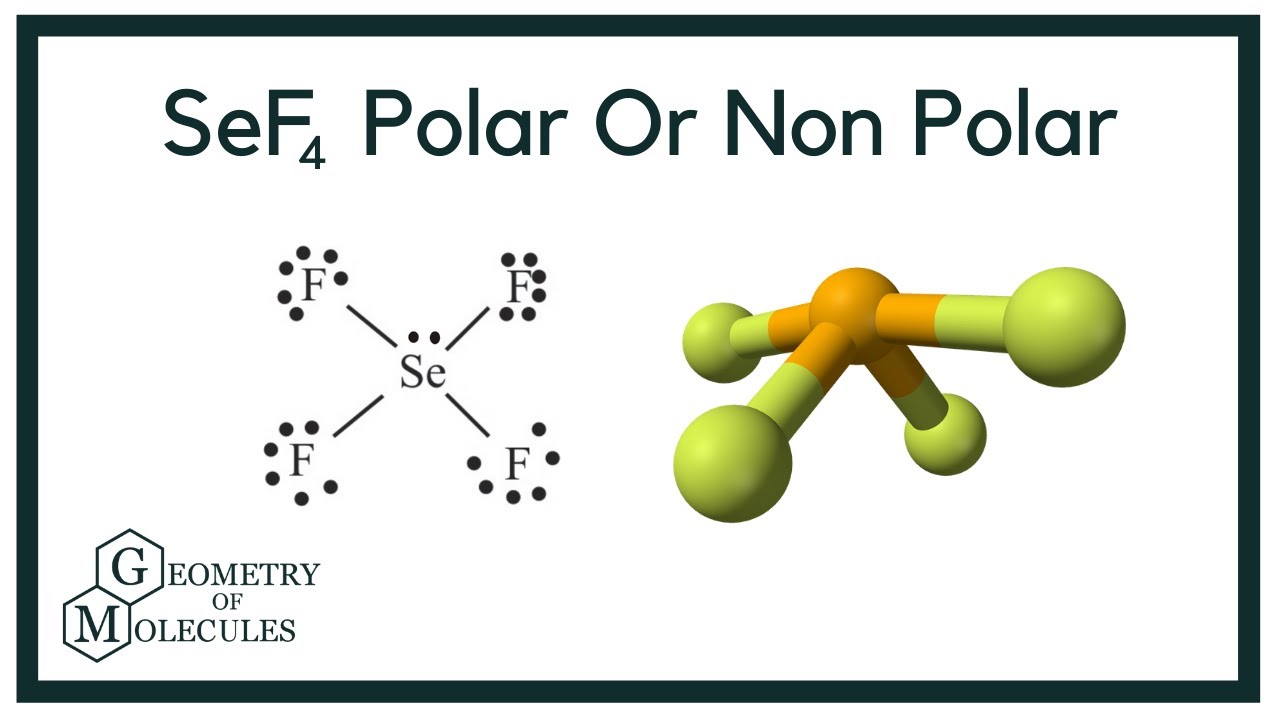
The molecular geometry of SeF4 is see-saw and electron geometry is trigonal bipyramidal this is because the selenium central atom has one lone pair on equatorial position and 4.

Sef4 molecular structure. Computed by Cactvs 34611 PubChem release 20190618 Exact Mass. SF4 is a dangerous substance that is frequently utilized in chemical and pharmaceutical businesses. Bond dipoles do not cancel.
The shape is like a seesaw. You can put these on the central Se atom. The way to determine the molecular geometry of SeF4 is to first draw the Lewis Dot Structure.
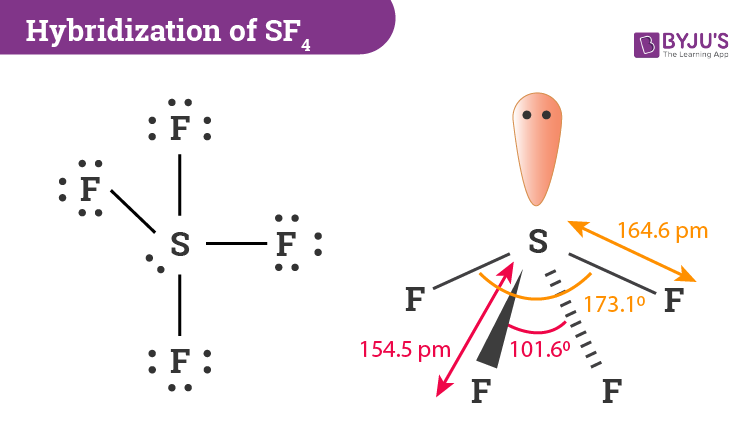
The central sulfur atom is linked to four fluorine atoms and contains one lone pair. We hope that this article was insightful enough and you got the basic idea about this interesting compound. The structure of SF 4 can therefore be anticipated using the principles of VSEPR theory.
Computed by Cactvs 34611 PubChem release 20190618 Hydrogen Bond Acceptor Count. One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. The bonds formed between two atoms are depicted using lines whereas the valence electrons not forming any bonds are shown by dots.
SeF4 is Lewis structure with Selenium which can hold more than 8 valence electrons. It is a see-saw shape with S at the center. Why is XeF2 linear and not bent.
A 5359935 B 3395079 C 2780431 MHz for 80 SeF 4 and A 5370448 B 3399281 C 2780416 MHz for 78 SeF 4. The Lewis structure for XeF2 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. Computed by PubChem 21 PubChem release 20210507 Hydrogen Bond Donor Count.
Laboratory Chemical Safety Summary LCSS Datasheet. Here in this post we described step by step method to construct SF4 molecular geometry. The corresponding molecular shape for AX2E3 is linear.
Consequently the molecule has two distinct types of F ligands two axial and two equatorial. What is the electron geometry of if4. A quick explanation of the molecular geometry of SF4 including a description of the SF4 bond anglesIt is important to note that you must first draw the corr.
SF4 has a trigonal bipyramidal molecular geometry. Lewis structure is a pictorial representation of the bonds and valence electrons in the molecule. Vapor is heavier than air.
This means that SeF4 has Trigonal Bipyramidal structure with 4 bond pairs and 1 lone pair. The shape generally resembles a see-saw and this shape is due to the repulsion in bonding and lone pairs of electrons. The correct molecular shape for XeF2 is linear.
SeF4 is Lewis structure with Selenium which can hold more than 8 valence electrons. The microwave spectra of two isotopes of selenium tetrafluoride SeF 4 have been recorded between 8000 and 40 000 MHz. So Hybridization sp3d2 the shape of the molecule is octahedral geometry but the geometry of the atom takes a square planar shape is due to iodine carries two lone pairs of electrons one above the.
Hence SF4 has a trigonal bipyramidal molecular geometry. Youll have a pair of electrons left over after filling octets of the F atoms. SF4 lewis structure comprises one sulfur and four fluorine atoms.
Does SeF4 obey octet rule. What is the the shape molecular geometry of nicl2. Since there are seven Fluorine F atoms it will be necessary.
Since there are seven Fluorine F atoms it will be necessary. This means that SeF4 has Trigonal Bipyramidal structure with 4 bond pairs and 1 lone Continue Reading You can guess the molecular structure of any molecule by knowing the no. The molecular geometry of SF4 according to its molecular formula and hybridization is trigonal bipyramidal.
Remember that Xenon can have more than 8 valence electrons. The Lewis structure of SF4 is the combination of 34 valence electron and 5 electron pairs around the Sulfur in which there are four bonding pairs and one lone pair. The valence electrons that participate in forming bonds are called bonding pairs of electrons.
Computed by Cactvs 34611 PubChem release 20190618 Rotatable Bond Count. The relevant bond distances are SF ax 1643 pm and SF eq 1542 pm. The rotational constants that predict the spectra are.
Drawing and predicting the SF4 molecular geometry is very easy.
Hybridization Of Sf4 Hybridization Of S In Sulfur Tetrafluoride
1 What Is The Lewis Structure For Sef 4 2 What Is Its Electron Geometry 3 What Is Its Molecular Geometry 4 What Is Its Hybridization 5 How Would You Classify It In
What Is The Molecular Geometry Of Sf4 Quora

Sef4 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

Determine The Molecular Geometry Of Sef4 Clutch Prep
Is Sef4 Polar Or Non Polar Selenium Tetrafluoride Youtube
Sef6 Lewis Structure How To Draw The Lewis Structure For Selenium Hexafluoride Youtube

Sef4 Lewis Structure How To Draw The Lewis Structure For Sef4 Youtube
Selenium Tetrafluoride F4se Chemspider
What Is The Molecular Geometry Of Sef4 How Is It Determined Quora
Sf4 Molecular Geometry Lewis Structure Bond Angles And Polarity

Consider The Molecule Sef4 A Draw The Lewis Structure B What Is The Hybridization Of Se C What Is The Electron Geometry D What Is The Molecular Geometry E What Degree Angles

Datei Selenium Tetrafluoride Svg Wikipedia

Sf4 Lewis Structure How To Draw The Lewis Structure For Sf4 Youtube

Datei Selenium Tetrafluoride Svg Wikipedia
Http Www Msubillings Edu Sciencefaculty Handouts Wiles Chem 20116 Solution 20set 202 Pdf

Type Of Hybridisation In Transition State Of Sef4 When It Undergoes In Hydrolysis

How Many Possible Fsef Bond Angles Are Present In Sef4 Molecule
