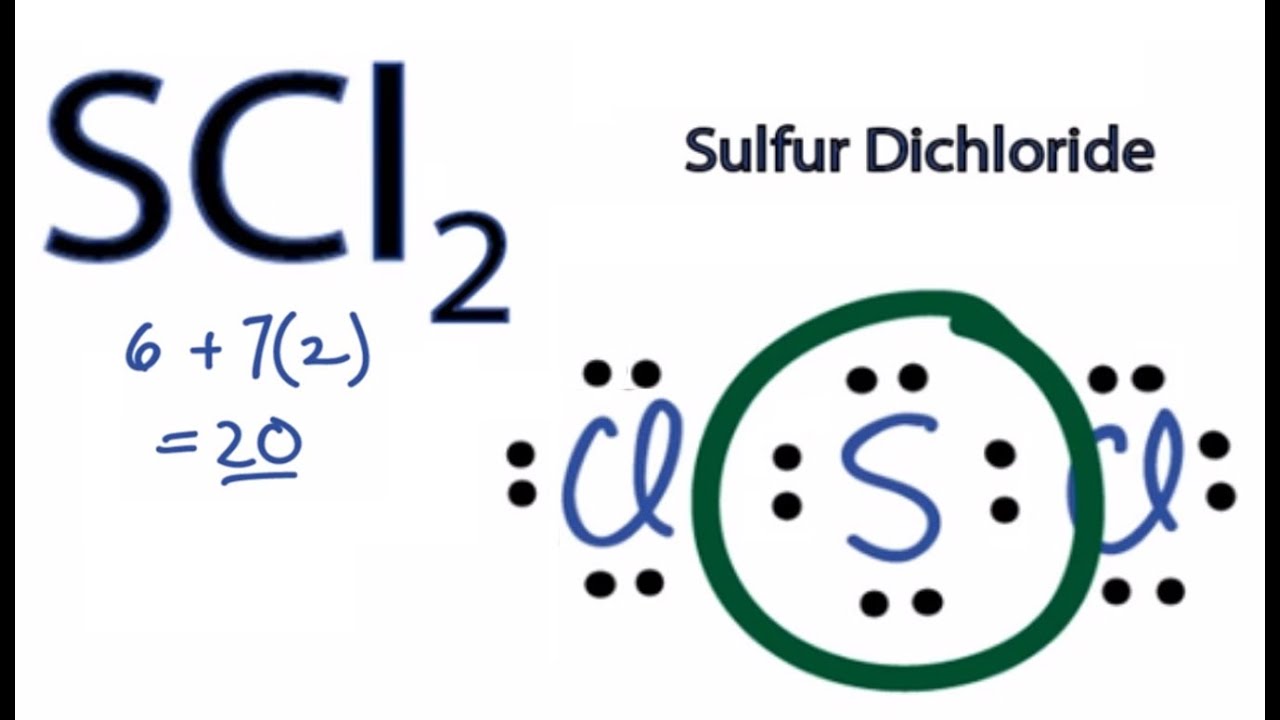
Sulfur Dichloride Lewis Dot Structure
Steps to draw electron dot structure or lewis structure of SCl2 Step 1. Sulfur is the center atom that is double bonded to the chlorine atoms.
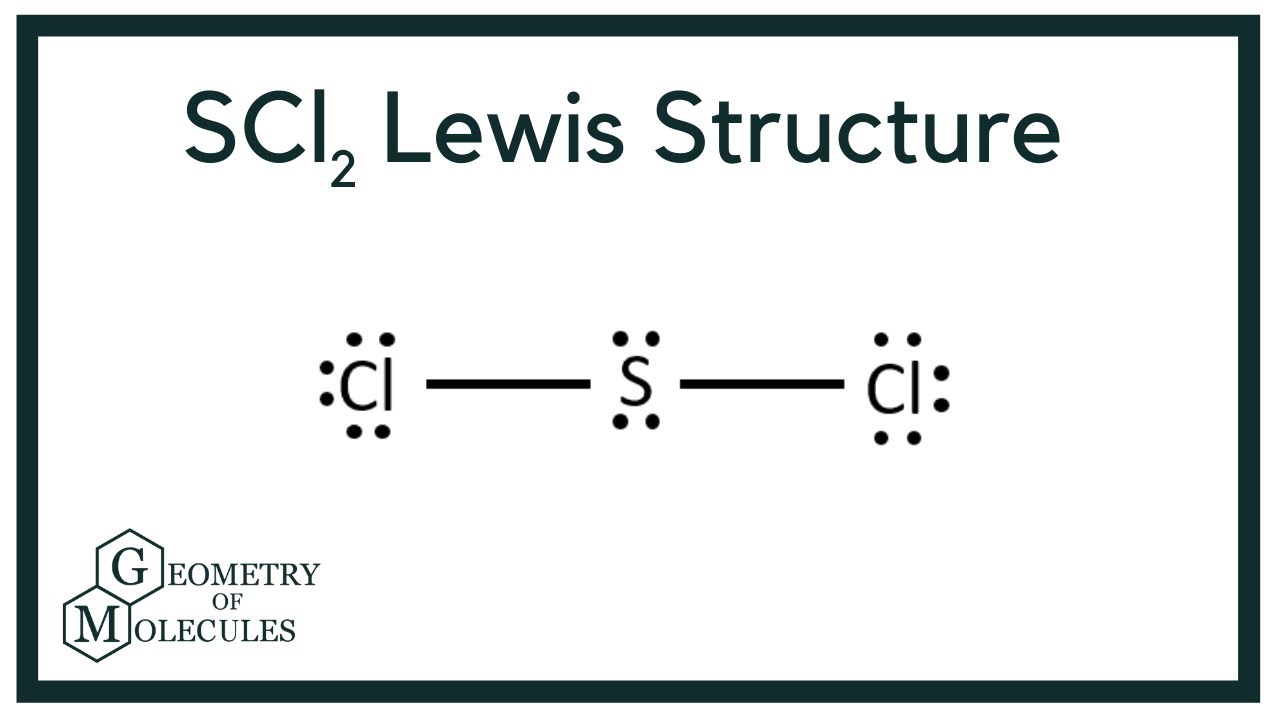
Scl2 Lewis Structure Sulfur Dichloride Youtube
Follow some steps for drawing the lewis dot structure of ICl2-1.
Sulfur dichloride lewis dot structure. To make bonds four pairs are needed so one pair remains alone. Which statement best describes the correct Lewis-dot structure. Count total valence electron in ICl2-.
Write the skeleton structure of the molecule. The sulfur atom here has two bonding pairs shown as horizontal lines and two lone pairs shown as two dots for each pair. Sulfur being the less electronegative atom than chlorine placed at the center in lewiss diagram and chlorine spaced evenly around it.
SCl2 lewis structure contains one sulfur and two chlorine atom. So weve used all 26 valence electrons in the S2CL2 Lewis structure. The key is to understand the steps and practice.
The product contains about 72 - 80 SCl2 residual S2Cl2 and Cl2. The shape of a molecule. The Lewis Structure of SCl2 sulfur dichloride has one sulfur single-bonded two each of two chlorine atoms.
For the SCl2 Lewis structure use the periodic table to find the t. 70 More Lewis Dot Structures. The physical properties of the molecule like boiling point surface tension etc.
Has a similar shape to water due to 2 lone valence pairs on the sulfur. This is the S2Cl2 Lewis structure. None of these require pi-bonding which is the method of formation for double and triple bonds.
Contaminated clothing and shoes should be removed and left at the worksite. Chlorine has 8 it has an octet. Draw the Lewis-dot structure for sulfur dichloride SC12.
A step-by-step explanation of how to draw the SCl2 Lewis Structure Sulfur Dichloride. Stabilized pure sulfur dichloride should contain minimum 98 SCl2. The sulfur atom here has two bonding pairs shown as horizontal lines and two lone pairs shown as two dots for each pair.
Sulfur dichloride lewis structure. Also the iodine central atom in ICl2- lewis structure violates the octet as it is holding more than 8 electrons in its octet shell. First you should count the total number of valence electrons in SCl2.
SF2 Lewis Structure Molecular Geometry Hybridization Polarity and MO Diagram. Molecular geometry lewis dot of sulfur dichloride scl 2 back 70 more lewis dot structures this cherry red liquid is the simplest sulfur chloride and one of the most common has a similar shape to water due to 2 lone valence pairs on the sulfur sulfur and oxygen are in. Sulfur Fluoride is a highly unstable inorganic compound.
Sulfur has 8 this Sulfur has 8 and then this Chlorine here also has a full outer shell with 8 valence electrons. Determine the total number of valenceelectrons. Hazardous Substances Data Bank HSDB Sulfur dichloride is sold in technical grade with a chlorine content of 66 - 70.
B and thanks for watching. Lewis Structures are important to learn because they help us predict. Every chemistry student has to learn how to draw Lewis Dot Structures.
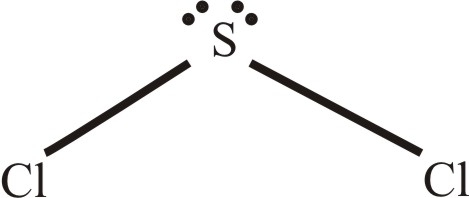
The sulfur atom has 4 lone pairs of electron while each chlorine atom has four lone pairs. Forms an angular or bent shape with a bond angle of 102 o. Use two valence electrons to form each bond inthe skeleton structure.
Sulfur and oxygen are in group 16 and bond similarly. It is singly bonded to each chlorine atom. This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or mercury fluoride.
In Uncategorized on February 12 2021 February 12 2021 Share Facebook Twitter Pinterest Email. How the molecule might react with other molecules. So thats the Lewis structure for S2CL2.
None of these require pi-bonding which is the method of formation for double and triple bonds. Try to satisfy the octets of the atoms bydistributing the remaining valence electrons as nonbondingelectrons. While sulfur dichloride does not burn easily it may ignite other combustible materials eg wood paper oil.
Sulfur is the center atom. This cherry-red liquid is the simplest sulfur chloride and one of the most common. With a molar mass of 70062 gmol this compound is made up of one Sulfur atom and two Fluoride atoms.
The Lewis Structure of SCl2 sulfur dichloride has one sulfur single-bonded two each of two chlorine atoms. Lets see how to draw the lewis dot structure of Iodine dichloride with easy steps.
Bond Angle Of Scl2 Lewis Structures
Solved Predict The Geometry Of Sulfur Dichloride Scl2 And The H Chegg Com
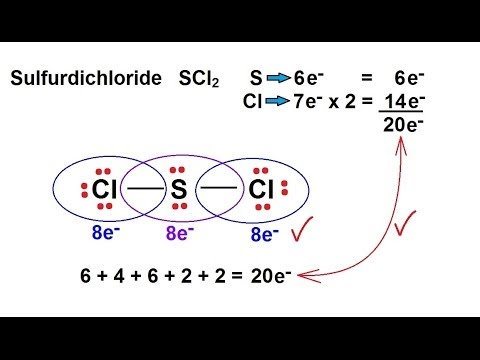
Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Youtube
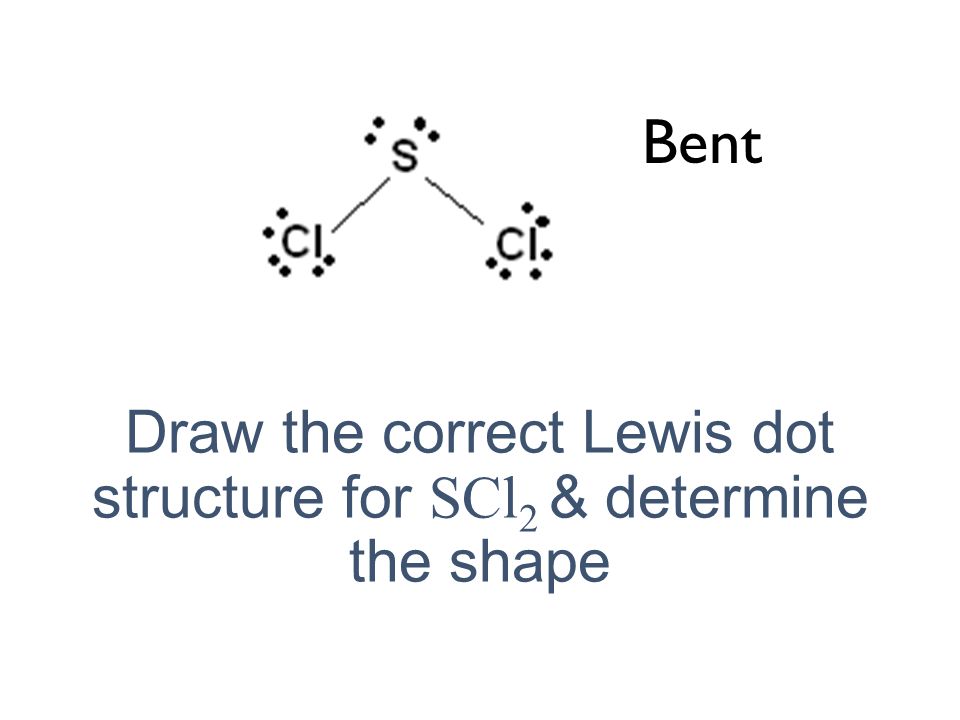
Draw The Correct Lewis Dot Structure For Nacl Ppt Video Online Download

Scl2 Sulfur Dichloride Molecular Geometry Bond Angles Electron Geometry Youtube

Is Scl2 Polar Or Nonpolar All About Scl2 Polarity

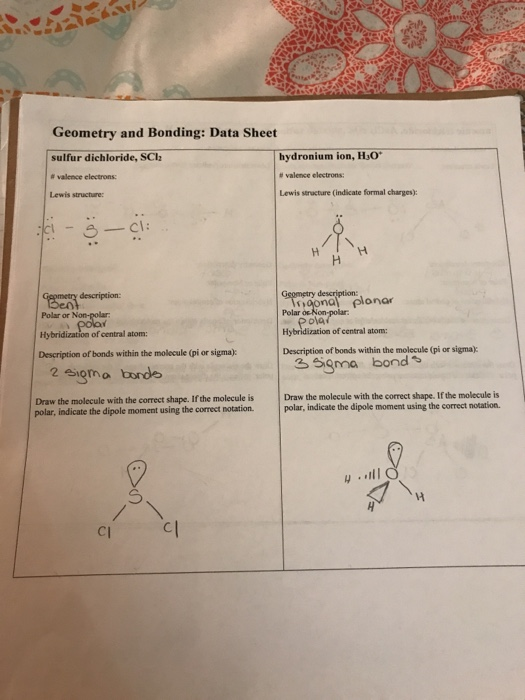
Geometry And Bonding Data Sheet Sulfur Dichloride Chegg Com

Scl2 Lewis Structure Molecular Geometry Or Shape Polarity Hybridization

Chemistry Chemical Bonding 9 Of 35 Lewis Structures Sulfur Dichloride Scl2 Youtube

Why Is Scl2 Polar I Have The Lewis Structure And From There I Can See That It Has Polar Bonds Because Of The Cl So Both S Cl Bonds Are Polar Bonds

Does Scl2 Have A Dipole Moment Clutch Prep
Valence Shell Electron Pair Repulsion
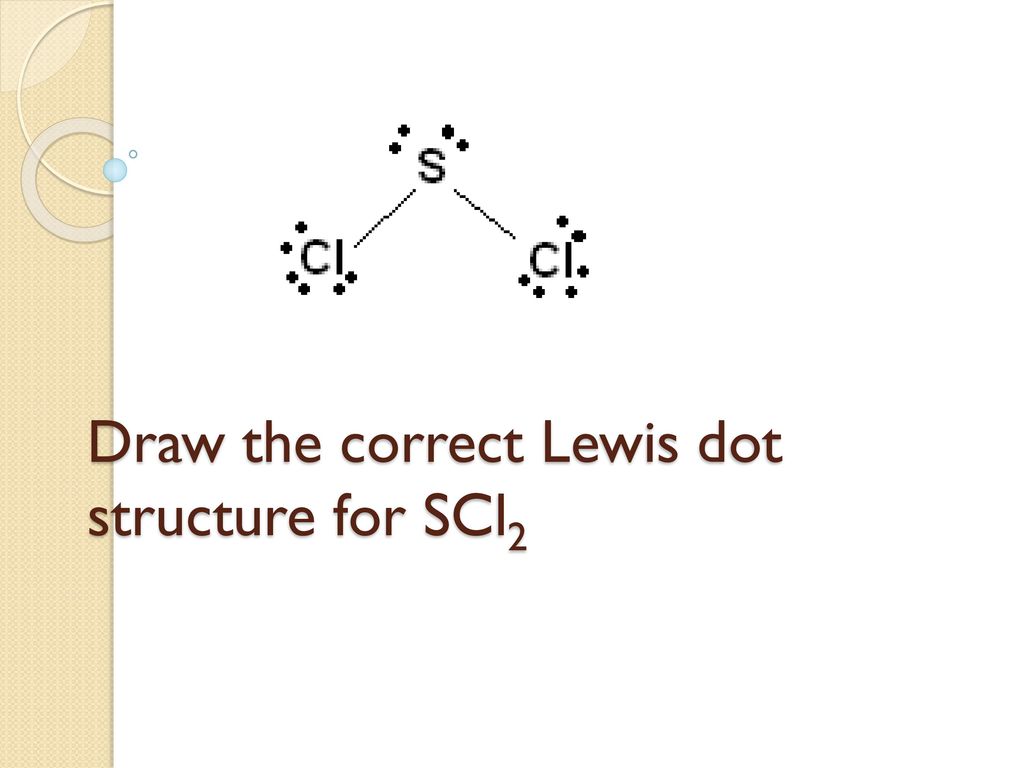
Types Of Bonding And Lewis Structures Ppt Download
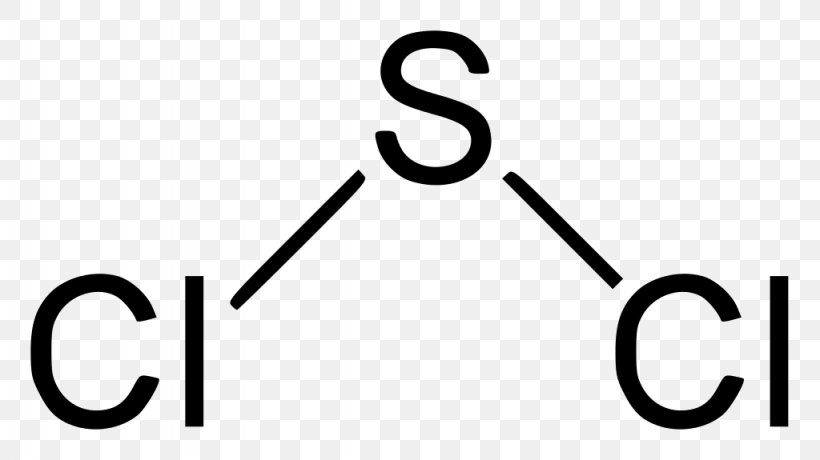
Disulfur Dichloride Lewis Structure Png 1024x575px Sulfur Dichloride Area Brand Calcium Chloride Chemical Bond Download Free

What Is The Name Of The Hybrid Orbitals Us Clutch Prep

The Lewis Diagram For Scl2 The Electron P Clutch Prep
Scl4 Lewis Structure How To Draw The Lewis Structure For Sulfur Tetrachloride Youtube

Is Scl2 Polar Or Non Polar Sulfur Dichloride Youtube