What Is The Electron Geometry Of Pcl5
Furthermore How do you find the molecular geometry Steps Used to Find the Shape of the Molecule Draw the Lewis Structure. What is the electron geometry of PCl5.

Hybridization Of Pcl5 Molecular Geometry Iit Jee Neet Cbse Youtube
As there are 5 bonds of Cl.

What is the electron geometry of pcl5. What is hybridization of PCl5 are all the P-Cl bonds same give reason. In addition to tetrahedral another common shape for AB4 molecules is square planar. An explanation of the molecular geometry for the PCl5 ion Phosphorous pentachloride including a description of the PCl5 bond angles.
As per the law of mass action Phosphorus pentachloride. To a first approximation these electron pairs arrange themselves in a tetrahedronwith ideal bond angles of 1095. What is the molecular geometry of SO3.
The molecular geometry of pcl5 is trigonal bipyramid with symmetric electron region distribution around the central atom. On carbon-carbon bond in C2H2 how do you compare the results. How many types of CL P-Cl bonds are present in PCl5.
The ideal bond angle for the Phosphorous pentachloride is 90 120 since it has a Trigonal bipryamidal molecular geometry. By signing up youll get thousands of step-by-step solutions to your homework questions. Phosphoruss electronic configuration in its ground state is 1s2 2s2 2p6 3s2 3p2 as the total number of valence electrons is 5.
Phosphorus Pentachloride or PCl5 is a compound formed by chemical elements Phosphorus Atomic number. A Phosphorus Pentachloride molecule consists of 1. What is the shape molecular geometry of PCl3 The molecular geometry of PCl 3 is trigonal pyramidal with asymmetric charge distribution on the central atom.
PCl5 has a trigonal bipyramidal structure due to the dsp3 hybridization. When it is in an excited state one of the electrons in the s-orbital moves to the d-orbital and the valence electrons of p orbitals get unpaired to move to the higher orbitals. Due to this trigonal bipyramidal structure the 2wo Cl-atoms of PCl5.
8-9 Polarity Review Structures Responses. 4 Zeilen What is the electron geometry of pcl5. What is the electron-pair geometry for p in pcl5 In order to continue enjoying our site we ask that you confirm your identity as a human.
What is the electron geometry of XeF2. What is the electron geometry of H2O. For this we need to do the following steps.
Linear with 22 valence electrons and 5 effective pairs around the central atom two of which are the Kr-Cl bonds the other three are lone pairs around Kr. What is the molecular geometry of SF6. What is the molecular geometry of PCl5.
Back to molecular geometries polarity tutorial. P and Chlorine Atomic number. It can be prepared by the action of dry chlorine on phosphorus trichloride.
And after connecting upper Cl with 3 in same plane Cl and down Cl with 3 in same plane Cl is make shape like an pyramid. What is the electron geometry of a AB4 molecule. Does PCl5 have regular geometry.
What is the shape molecular geometry of PCl3 The molecular geometry of PCl 3 is trigonal pyramidal with asymmetric charge distribution on the central atom. Geometry of PCl5 is trigonal bipyramidal. 5 x Cl contibute.
However the lone pairs on the central atom in the molecule leads to a bent molecular geometry. So it makes triangle when 3 are in same plane. Phosphorus pentachloride is known to have the salt-like structure in the crystalline state and to be partly dissociated in solution especially in polar solvents such as nitrobenzene.
Each Hydrogen atom has only one electron which is also its valence electron. PCl5 has two binding angles in a trigonal bipiramidal form 5 bond pairs Disposing the PF3 PCl3 PBr3 and PI3 in increasing order of connection angle with suitable reason. Thank you very much for your cooperation.
PCL5 has five electron domains without lone pairs of electrons on its central atom. Hence the shape formed is called trigonal bipyramidal. It has an regular geometry.
Phosphorus Trichloride has a trigonal pyramidal shape as the electrons are arranged in a tetrahedral geometry. What is the electron geometry of pcl3 Best answer. You will discover that Se will have a lone pair.
Count the number of electron groups and identify them as bond pairs of electron groups or lone pairs. Phosphorus pentachloride is a pale greenish-yellow solid with the formula PCl 5. 3 in same plane and one in upper and another in down palne.
SO2 has four electron domains leading to a tetrahedral electron domain geometry according to valence shell electron pair repulsion theory. What is the electron geometry of ClF3. The molecular geometry shape of NF3 is _____.
Determine the central atom in this molecule.

Pcl5 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Is Pcl5 Polar Or Nonpolar Techiescientist

Pcl5 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

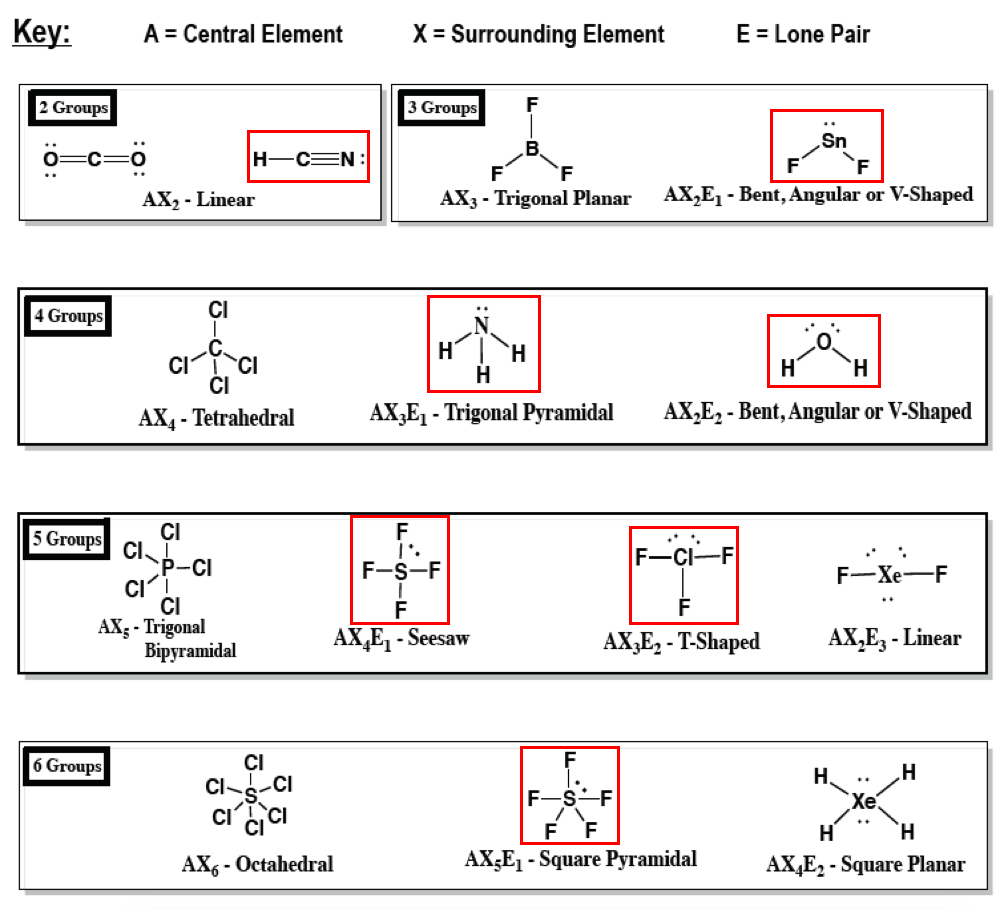
Molecular Geometry Predicted By Vsepr Ppt Download
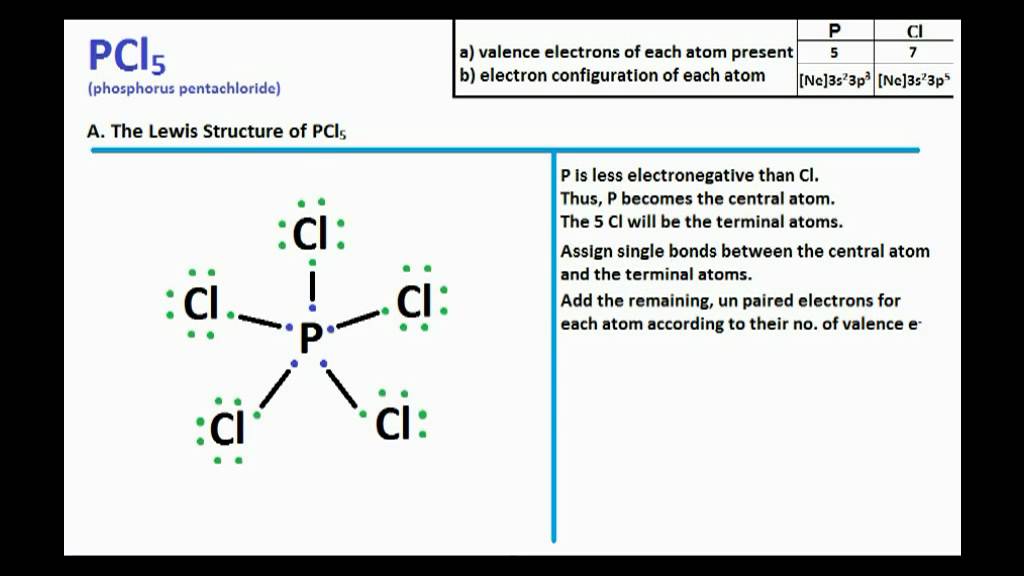
Pcl5 Lewis Structure And Molecular Geometry Youtube

What Is The Molecular Geometry Of Pcl5 Quora
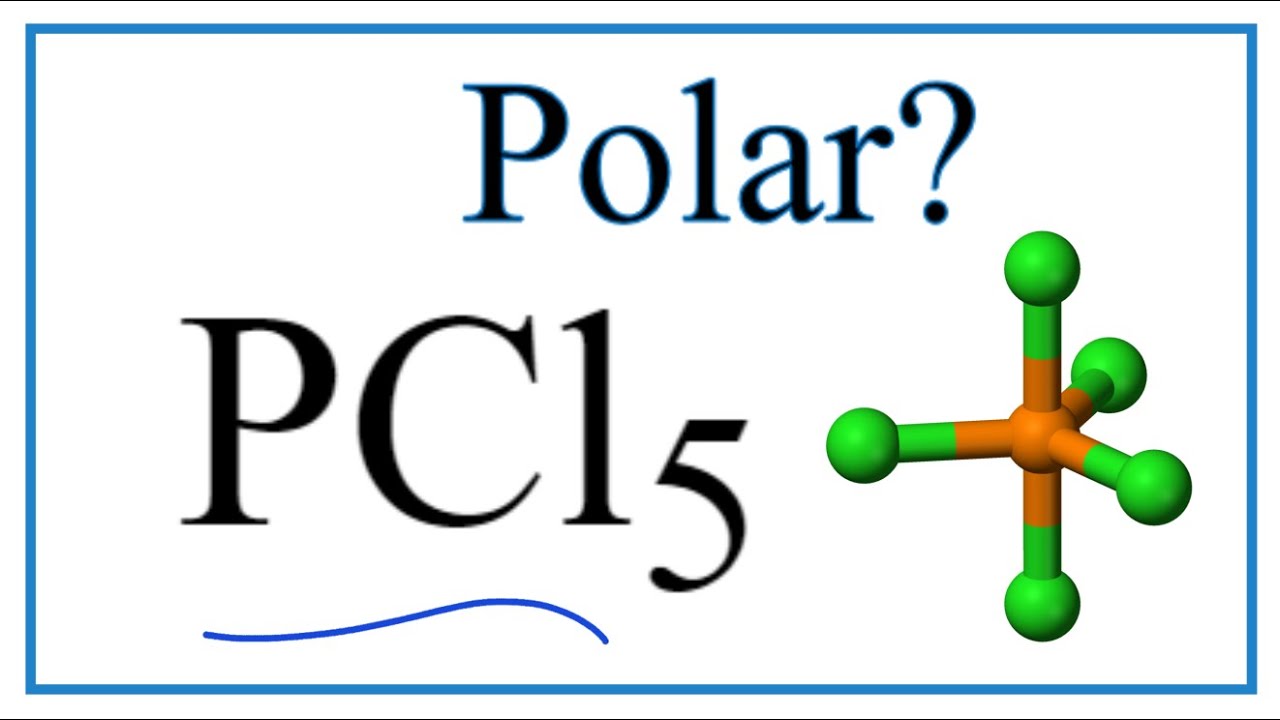
Is Pcl5 Polar Or Nonpolar Phosphorous Pentachloride Youtube
What Is The Molecular Geometry Of Pcl5 Quora
The Molecule Pcl5 Is Observed Not To Have Clutch Prep

What Is Trigonal Bipyramidal Geometry Brainly In

Pcl5 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
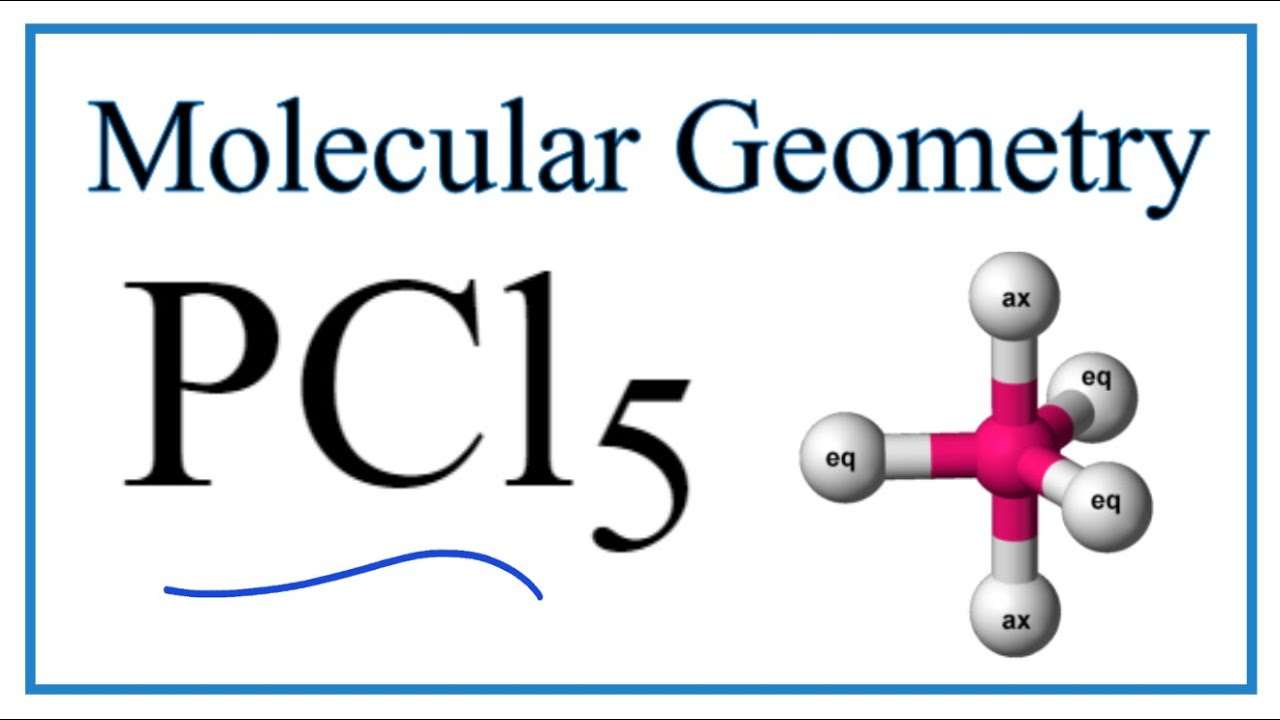
Pcl5 Phosphorous Pentachloride Molecular Geometry Bond Angles Youtube
Explain The Structure Of Pcl3 And Pcl5 Chemistry Topperlearning Com C9yvmoxx

Pcl5 Villanova College Chemistry Blog

Pcl5 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
What Is The Structure Of Pcl5 Quora

Pcl5 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Valence Shell Electron Pair Repulsion Theory Vsepr Chemistry Study Material Emedicalprep Com Emedicalprep
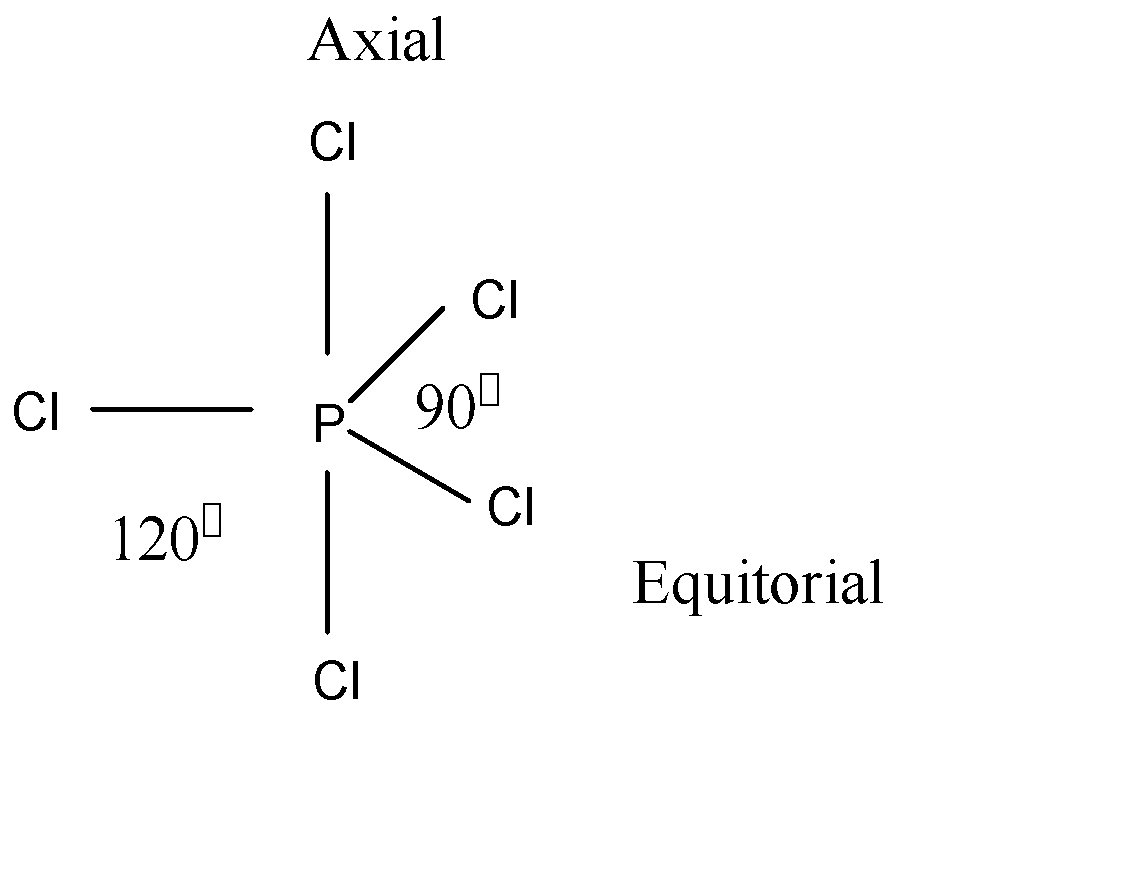
Cl P Cl Bond Angles In Pcl5 Molecule Is A 120 Circ Class 11 Chemistry Cbse
