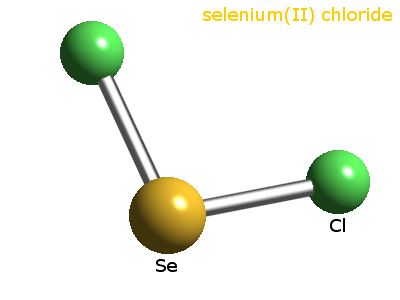
What Is The Molecular Shape Of Secl2
The central atom has 4 pairs of electrons 2 bonding pairs and 2 lone pairs so SeCl2 has a bent or V-shaped structure. What is the Lewis structure of SeCl2.

Scl2 Sulfur Dichloride Molecular Geometry Bond Angles Electron Geometry Youtube
Start with the molecules Lewis structure which is drawn like this.
What is the molecular shape of secl2. What is the shape of SeCl2. The molecular shape of the C_2Cl_2 molecule is linear. The electron pair geometry of SCl2 is tetrahedral as it determined by the steric number and S molecular central atom has a steric number that is equal to 4.
Secl2secl2 lewis structuresecl2 namesecl2 polarityscl2 shapesecl2 compound namesecl2 compoundsecl2 polar or nonpolar. Selenium dichloride is a covalent molecule with a central selenium atom. No information is available on the carcinogenic effects of chlorine in humans from inhalation.
7896 354532 Percent composition by element. From this we can describe the molecular geometry. Specifically for SeCl2 the total number of valence electrons are 6 Se 27 for 2 Cl atoms for at total of 20.
The disclosed method is suitable for producing a SiGe-on-insulator structure. Thus its Lewis structure must account for 22 valence electrons. All 20 valence electrons 6.
Chlorine is a commonly used household cleaner and disinfectant. Chlorine is a potent irritant to the eyes the upper respiratory tract and lungs. All 20 valence electrons 6.
What Is The Molecular Shape Of SeCl2. It is important to remember that Lewis structures are not meant to convey geometry so it would be wrong to assume that the molecule is linear just by looking at this particular Lewis structure. O Linear O Angular Or Bent O Trigonal Planar Tetrahedral None Of The Above What Is The Electronic Geometry Of A Bromate Bro3 Ion.
What are the Advantages of indirect cold water system over direct cold water system. Next Question Search your questions here. SCl2 molecular geometry is very similar to H2O but its bond angle is slightly lower than H2O because of the lone pair.
Shape and conformation are important for understanding intermolecular interactions and several structure-based. Molar mass of SeCl2 149866 gmol. The VSEPR model can be used to predict the shapes of many molecules and polyatomic ions but it gives no information about bond lengths.
This compound is also known as Selenium Dichloride. As Se is both further down and further to the left on the periodic table. Treatment of SCl2 with primary amines gives sulfur diimides.
Starting with its Lewis structure the C_2Cl_2 molecule has a total of 22 valence electrons 4 from each of the two carbon atoms and 7 from each of the two chlorine atoms. Favorite Answer Sulfur has six valence electrons plus 14 for two chlorines for a total of 20. Question Is SeCl2 polar or nonpolar.
What is the geometry shape around each carbon atom. Chronic long-term exposure to chlorine gas in workers has resulted in respiratory effects including eye and throat irritation and airflow obstruction. SCL2 molecular geometry or shape The bond angle of SCl2 is approx 103º.
Name Email Website Each shape has a name and an idealized bond angle associated with it. SCl_2 has a bent molecular geometry with bond angles of approximately 103 and a bond lenght of 201 pm. SeCl2 molecular weight.
SeCl2 What is the molecular shape around the central atom is it polar and does the molecule have any degenerate resonance forms. Answer SeCl2 is Polar What is polar and non-polar. However the lone electron will slightly distort the bond angles and push the bonded pairs away making the angles slightly less than 90 and 120 degrees.
So the two carbon atoms are bonded to the two chlorine atoms through a single bond and through a. Convert grams SeCl2 to moles or moles SeCl2 to grams. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance.
Get 11 help now from expert Chemistry tutors. Selenium Se contains 6 valence electrons on its own as a main group 6A element. O Bent O Linear Tetrahedral Trigonal Planar Trigonal Pyramidal What Is The Electronic Geometry Of NH2.
Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Expert Answer 100 1 rating Previous question Next question Get more help from Chegg. It will donate 2 of these valence.

Determine The Number Of Bonding Electrons And The Number Of Nonbonding Electrons In The Structure Of Brainly Com

Sample Exercise 9 1 Using The Vsepr Model Ppt Download
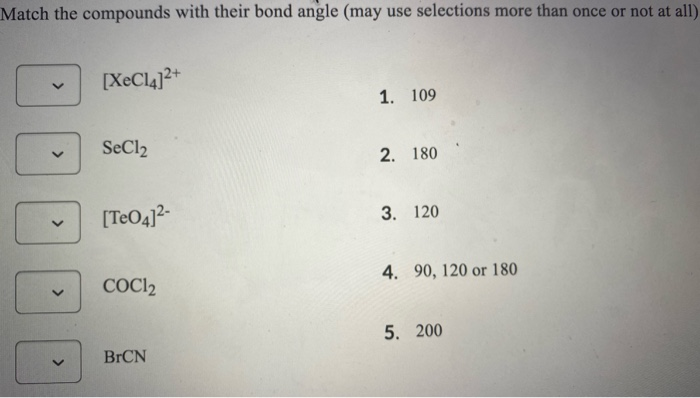
Match The Compounds With Their Bond Angle May Use Chegg Com

Wn Tecl2 Lewis Dot Structure Polar Or Nonpolar Bond Angle Molecular Geometry And Hybridization
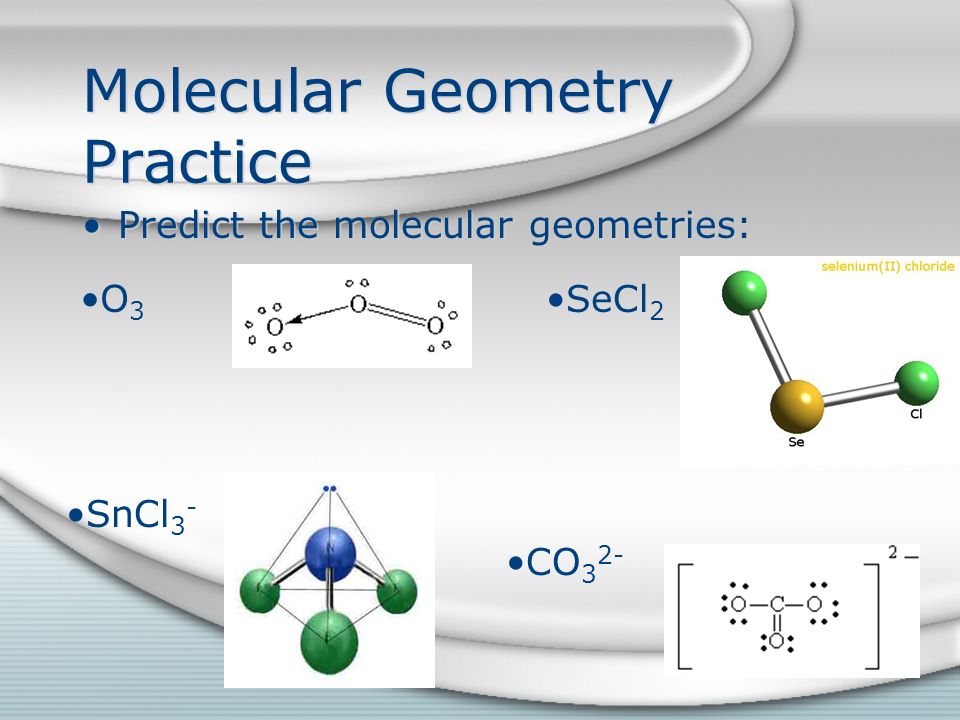
Molecular Geometry Bonding Theories Ppt Video Online Download

Determine The Number Of Bonding Electrons Clutch Prep

How To Draw The Lewis Dot Structure For Secl2 Selenium Dichloride Youtube

How To Find The Lewis Structure For Secl2 Quora

Molecular Geometry And Bonding Theories The Properties Of

5 Draw The Best Lewis Structure For The Following Molecul Clutch Prep
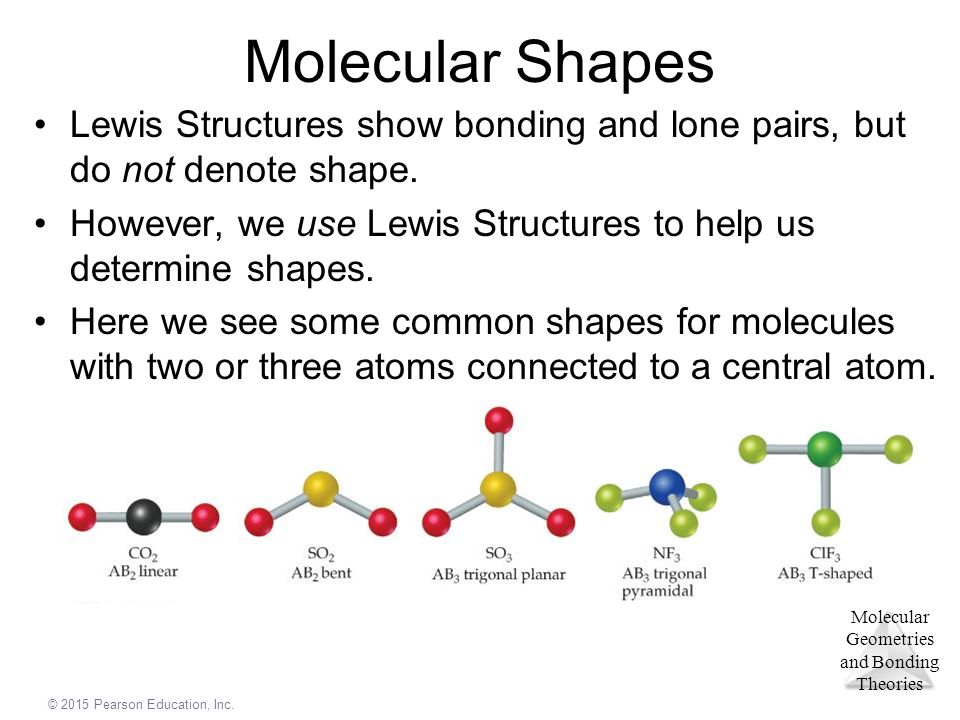
Chapter 9 Molecular Geometry And Bonding Theories Ppt Video Online Download
Chemistry The Central Science Chapter 9 Section 2
Selenium Dichloride Cl2se Chemspider

Drawing Lewis Structures And Vsepr Draw Basic Lewis Dot Structures Of Atoms And Compounds Using Vsepr Predict Bond Shape From Electron Arrangement Ppt Download
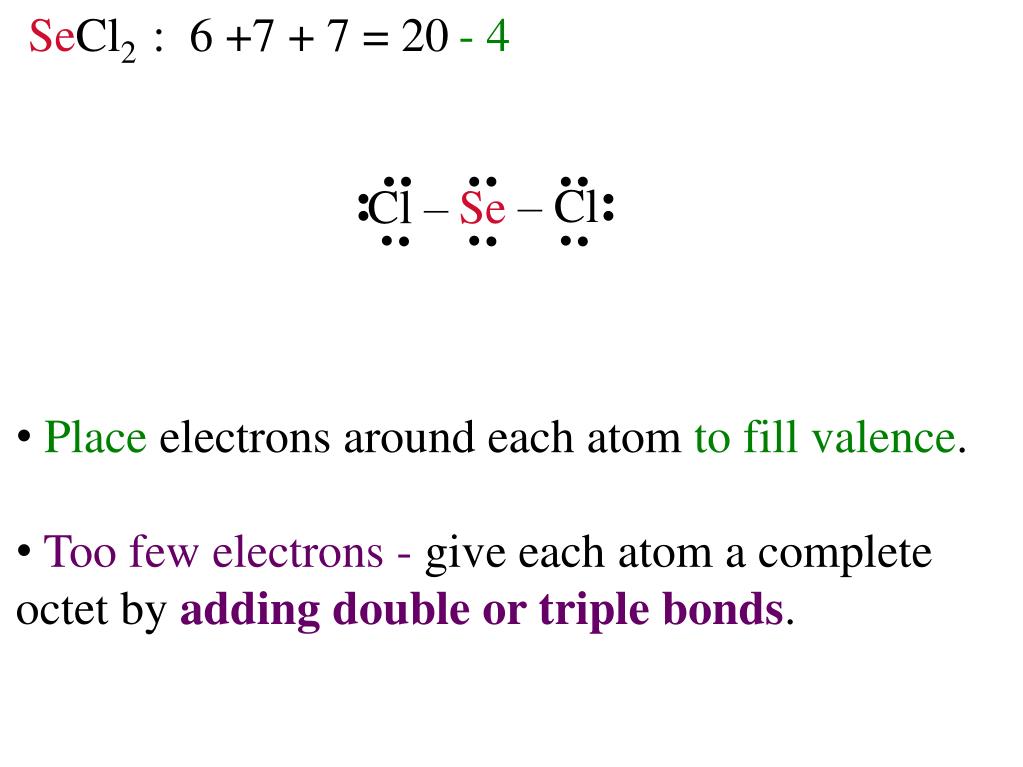
Secl2 Lewis Structure Shefalitayal
Webelements Periodic Table Selenium Selenium Dichloride

How To Draw The Lewis Dot Structure For Secl2 Selenium Dichloride Youtube
Http Site Iugaza Edu Ps Mlatif Files 2014 09 Molecular Geometry And Bonding Theories Chapter 9 Chema13011 Pdf
Http Zfn Mpdl Mpg De Data Reihe B 38 Znb 1983 38b 1072 Pdf
