What Is Wrong With The Following Lewis Dot Structure For Co2
Because the electronegativity difference between the two is not large enough for one atom to strip an electron from the other. Enter The Number Of Bonding Electrons Followed By The Number Of Nonbonding Electrons Separated By A Comma In The Dot Structure.

Makethebrainhappy The Lewis Dot Structure For Co2
So that first one is um you know out pretty quickly.

What is wrong with the following lewis dot structure for co2. So to complete their octet each oxygen makes 2 bonds and carbon makes 4 bonds. Essay answers are limited to about 500 words 3800 characters maximum including spaces 3800 Characters. So to complete their octet each oxygen makes 2 bonds and carbon makes 4 bonds.
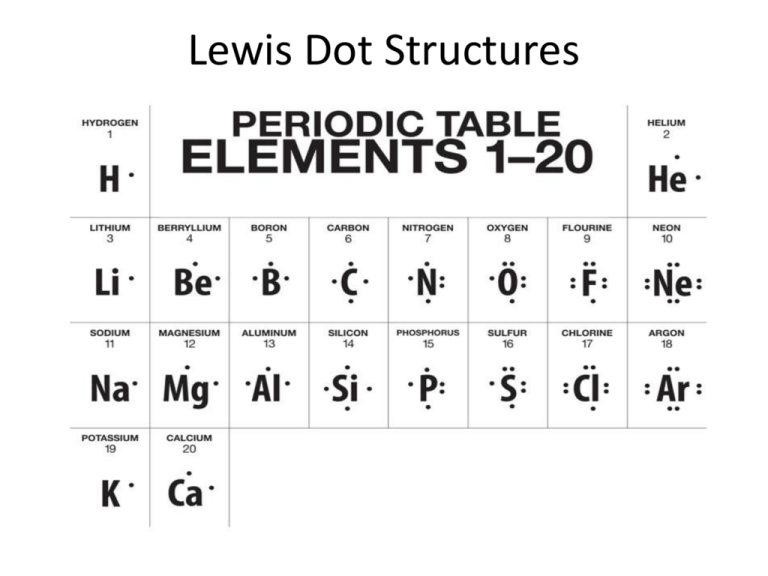
Chemical Bonding and Molecular Structure. The Lewis dot structure is drawn with letters that represent the atoms of the element and then a number of dots or dashes surrounding these letters. When drawing Lewis dot structures what should you do if you run out of electrons before all atoms are satisfied.
Try double or triple bonds. Then draw the correct structure. Draw Nonbonding Electrons Using The Dot Notation And Bonding Electrons As A Bond.
The lewis dot structure of CO2 gives it some unique properties. Drawing Lewis structures with O-O single bonds because these structures are very unstable. Carbon being lesser electronegative is the central atom as shown in the figure.
Whats Wrong With This Picture. Drawing Lewis structures where one or more atoms does not have a full valence shell. In CO2 Lewis structurethere are four lone pairsEach oxygen atom has two lone pairs around itThe carbon atom doesnt have any lone pair.
We will use this later in the class to make very reactive reagents. Basics of Chemical Bonding. Draw The Main Lewis Structure Of NOF.
A step-by-step explanation of how to draw the NI3 Lewis Dot Structure Nitrogen TriiodideFor the NI3 structure use the periodic table to find the total num. These properties in addition to its small state makes it so that carbon dioxide has a low melting point and is mostly in the gaseous phase at STP Standard Temperature and Pressure. Unstable and they do exist.
They dont have any crazy formal charges. Lewis structure is defined as a structure in which the electrons are represented by dots. And they have the correct number of Valence electrons that the structure.
And then the second the second and the third Lewis structures or both possible they were not breaking any of the acted rules. H H C H H. Carbon and oxygen has 4 and 6 electrons respectively in their outermost shell.
Lone pairs are not involved in covalent bonding. This Lewis structure is wrong because hydrogen atom cannot form bonds with two atoms. How To Read A Lewis Dot Structure.
Describe what is wrong with each of the following Lewis dot structures. Therefore it is nonpolar and relatively unreactive. Incorrect Lewis dot diagram is given by.
Lewis dot structure f Essay answers are limited to about 500 words 3800 characters maximum including spaces 3800 Characters remaining Submit My Answers Give Up Part E How many valence electrons does carbon have. Again these structures will be very reactive ie. If oxygen with an electronegativity of 34 were to share electrons with lithium the electronegativity of 10 what kind of bond would form.
Thus option B represents the correct structure of C O2. The other three pairs of electrons on each chlorine atom are called lone pairs A pair of electrons in a Lewis structure that is not involved in covalent bonding. Dots can be used to represent the shared electrons within the bonds of the atoms but dashes can be used.
The O-O single bond is very weak about 13 of a C-C and will break very readily. In CO2 Lewis structurethe carbon atom follows the octet rule and two oxygen atoms also follow the octet rule. H H C.
A The Lewis structure of the molecule is as follows. A step-by-step explanation of how to draw the Al2O3 Lewis Dot StructureFor Al2O3 we have an ionic compound and we need to take that into account when we dra. It recognizes the three-dimensional shape of a specific molecule.
H H H. Since there are no lone pairs on the atom it is a linear structure which makes the charges cancel it. Therefore the correct Lewis structure would be.
Step 1 of 5. Thus option B represents the correct structure of C O2. Determine The Number Of Bonding Electrons And The Number Of Nonbonding Electrons In The Structure Of BeF2.
Carbon being lesser electronegative is the central atom as shown in the figure. A step-by-step explanation of how to draw the CH2O Lewis Dot StructureCarbon C is the least electronegative atom in the CH2O Lewis structure and therefore. If both electrons in a covalent.
Why do covalent bonds form between two atoms. Lets go over the Lewis structure and find out how to interpret this representation of carbon dioxide. H H H.
The electron pair being shared by the atoms is called a bonding pair A pair of electrons in a Lewis structure that is shared by two atoms thus forming a covalent bond. A step-by-step explanation of how to draw the N3- Lewis Dot StructureFor the N3- Lewis structure use the periodic table to find the total number of valence. H H H.
However there is probably a better Lewis structure. Carbon and oxygen has 4 and 6 electrons respectively in their outermost shell. Part D - Explain what is wrong with the following statement.
Incorrect Lewis dot diagram. The term valence refers to an outer bonding electron.

Lewis Dot Structure Easy Hard Science

Write The Electron Dot Structure Of Co2 H2o H2s Propane F2 Ch4 Brainly In
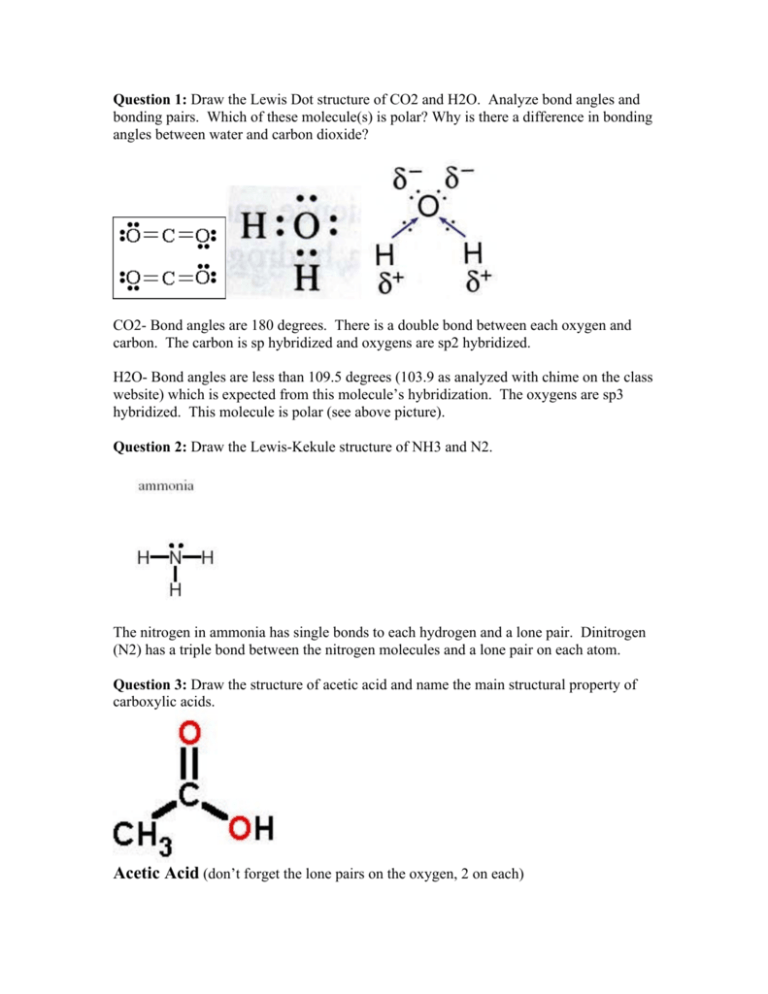
Question 1 Draw The Lewis Dot Structure Of Co2 And H2o Analyze

Co2 Lewis Structure Easy Hard Science

O2 Lewis Structure Easy Hard Science

Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube
Co2 Lewis Structure Molecular Geometry And Hybridization

Co2 Lewis Structure Carbon Dioxide Youtube

Co2 Lewis Structure Molecular Geometry And Hybridization Molecular Geometry Molecular Lewis

Ccl4 Lewis Dot Structure High School Chemistry Lewis Electron Configuration

Co2 Lewis Structure Easy Hard Science

Oh Lewis Structure How To Draw The Lewis Dot Structure For The Hydroxide Ion Youtube

Lewis Structures Practice For Interactive Notebooks Chemistry Lessons Teaching Chemistry Chemistry Education

Sf2 Molecular Geometry Lewis Structure Polarity And Bond Angles Molecular Geometry Molecular Bond

Lewis Dot Structure Easy Hard Science
Does It Matter Where You Put The Dots On A Lewis Structure Quora

Co2 Lewis Structure Carbon Dioxide In 2021 Carbon Dioxide Lewis Molecules

What Would Be The Electron Dot Structure Of Carbon Dioxide Which Has Formula Co Sub 2 Sub Chemistry Q A