Brf3 Lewis Structure Bond Angle
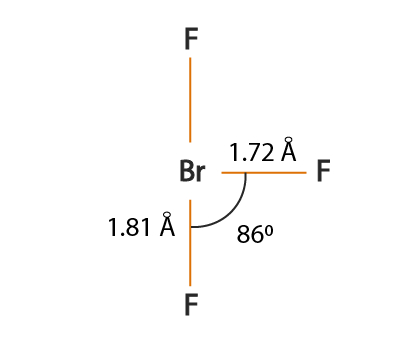
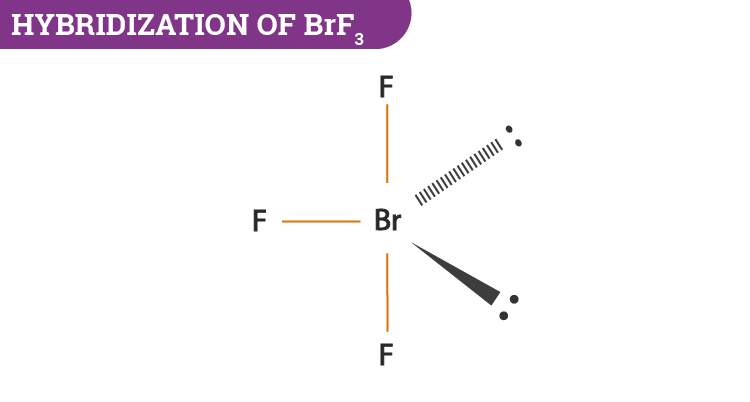
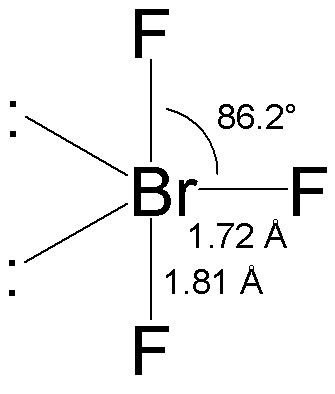
BrF3 has a T-shaped or trigonal bipyramidal molecular geometry as mentioned above with a bond angle F-Br-F of BrF3 is 862 which is somewhat less than the normal 90. All you would have to say is you dont need to know this exact bond angle all you need to know is that the electronic geometry is AX4 so technically its tetrahedral.

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
The angle is generated because the electron pairs repel one other more strongly than the Br-F bonds.

Brf3 lewis structure bond angle. Use the average bond energies to calculate the enthalpy change AH for the following reaction. BrF3 Bond angle. Bromine trifluoride chemical formula is BrF3.
The geometry of molecule of BF3 is Trigonal Planar With the reference of Chemistry Trigonal Planar is a model with three atoms around one atom in the middle. Drawing BrF3 Lewis Structure is very easy to by using the following method. Write the Electron Geometry Molecular Geometry Approximate bond angle Bond type ionic covalent polar covalent nonpolar Is BrF3 polar.
Order0629___ Top of page. The bond angles are compressed relative tothose in a perfect trigonal bipyramid due to lone pairs spreading out more in space than bonded pairs. Angle1381 deg___ Top of page.
So the hybridization of the BrF3 molecule is sp3d. BrF 3 molecular geometry is said to be T-shaped or Trigonal Bipyramidal with a bond angle of 862 o which is slightly smaller than the usual 90. Order0807___ between BR1 and F4.
The angle is formed due to the electron pairs repulsion which is greater than that of the Br-F bonds. BrF 3 molecular geometry is said to be T-shaped or Trigonal Bipyramidal with a bond angle of 862 o which is slightly smaller than the usual 90. Subsequently question is is BrF3 polar or non polar.
The bond angle of bromine trifluoride is in a T-shaped form that is slightly smaller than 90 degrees for the lines. The repulsion created by the electron pairs is higher than that of the Br-F bonds resulting in this angle. This happens due to the high electronegativity power of halogen F and also due to the influence of two lone pairs that push the bonds somewhat to make the angle less than 90 degrees.
But here the bond angles are reduced to around 86 degrees which is. Best Lewis Structure The Lewis structure that is closest to your structure is determined. After determining how many valence electrons there are in BrF 3 place them around the central atom to complete the octets.
It bent the shape of the BrF3 molecule. The ideal bond angle is 1095 but because that lone pair is there all youd have to really say is you. Drawing the Lewis Structure for BrF 3.
BrF3 Bond angle BrF3 molecular geometry is said to be T-shaped or trigonal bipyramidal as discussed with a bond angle of 862 which is slightly smaller than the usual 90. Of BrF 3 and determine the bond angle between an equatorial F atom and an axial F atom Draw the Lewis structure of BrF3 and determine the Bromine trifluoride BrF3 PubChem April 21st 2019 - BROMINE TRIFLUORIDE is a colorless to yellow fuming liquid with a pungent odor Solidifies at 48F Very toxic by inhalation and corrosive to metals and. Between BR1 and F2.
Angle1103 deg___ for F4-BR1-F2. BrF3 has a T-shaped or Trigonal Bipyramidal molecular geometry with a bond angle of 862 which is somewhat less than the typical 90. Its like peripheral atoms all in one plane as all three of them are similar with the 120 bond angles on each that makes them an equilateral triangle.
There are a total of 28 valence electrons for the BrF 3 Lewis structure. Drawing the Lewis Structure for BrF 3. This angle formed due to the repulsion generated by the electron pairs which is greater than that of the Br-F bonds.
BrF3 or bromine trifluoride is a highly polar. Because of 5 regions of high electron density three Br-F covalent bonds and two lone electron pairs - bromine trifluoride. Your email address will not be published.
Order0627___ between BR1 and F3. Therefore we have a bent T-shape for the BrF3 molecule. For the BrF 3 Lewis structure calculate the total number of valence electrons for the BrF 3 molecule.
Here in this post we described step. For the Lewis Structure of the BrF3 identify how many valence electrons this compound has.

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Solution Draw The Lewis Structure Of Brf Chemistry

Brf3 Polarity Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules
What Is The Hybridization Of Brf3 Quora

Brf3 Molecular Geometry Youtube

Hybridization Of Brf3 Bromine Trifluoride Youtube
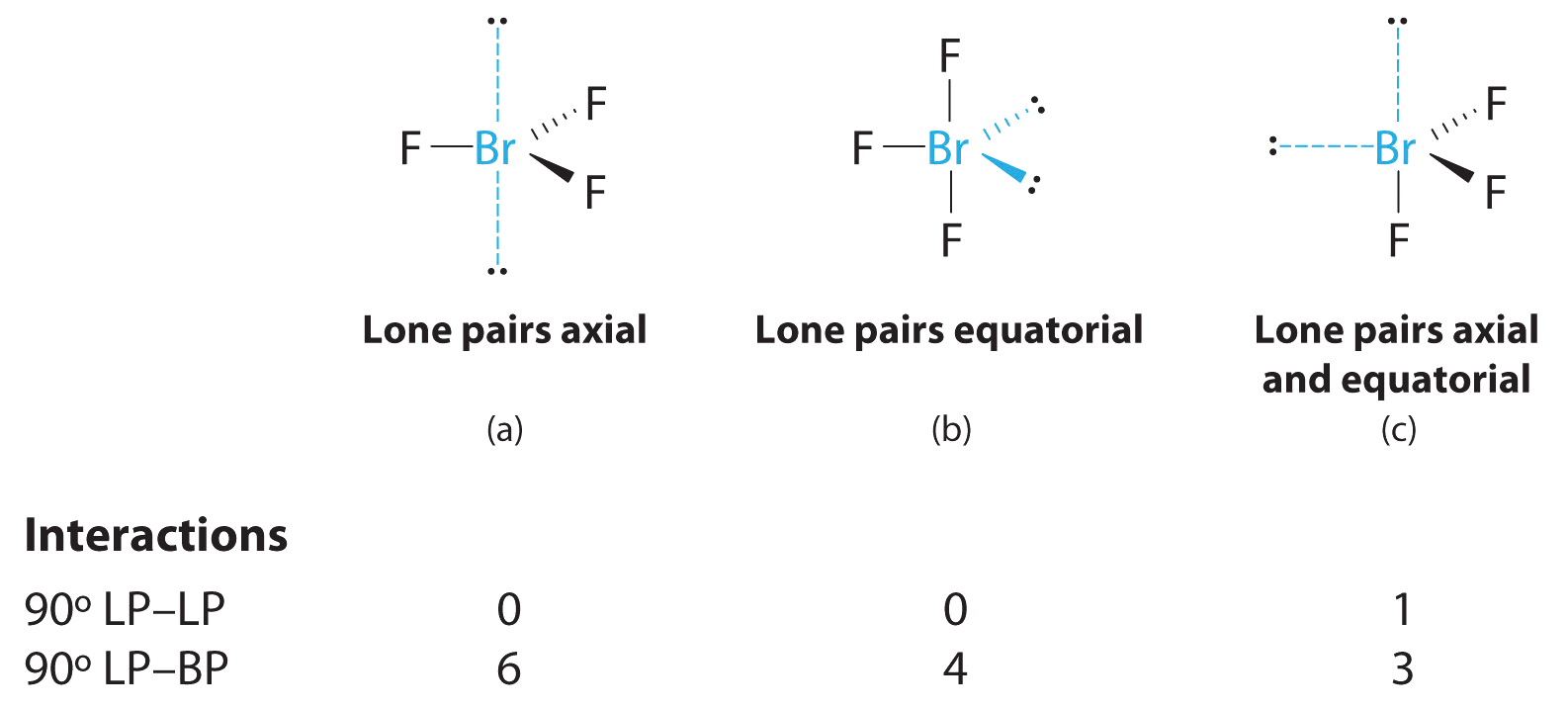
10 3 Vsper Theory The Effect Of Lone Pairs Chemistry Libretexts
Brf3 Bromine Trifluoride Molecular Geometry Bond Angles Youtube

Brf3 Polarity Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules
Hybridization Of Brf3 Hybridization Of Br In Bromine Trifluoride

Brf3 Lewis Structure Draw The Bromine Trifluoride Dot Structure Geometry Of Molecules

Whats The Molecular Geometry Of Asf3 Ch3 Brf3 Clo3 Xef2 Bro2 Study Com
Brf3 Lewis Structure Draw The Bromine Trifluoride Dot Structure Geometry Of Molecules
Brf3 Polarity Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules
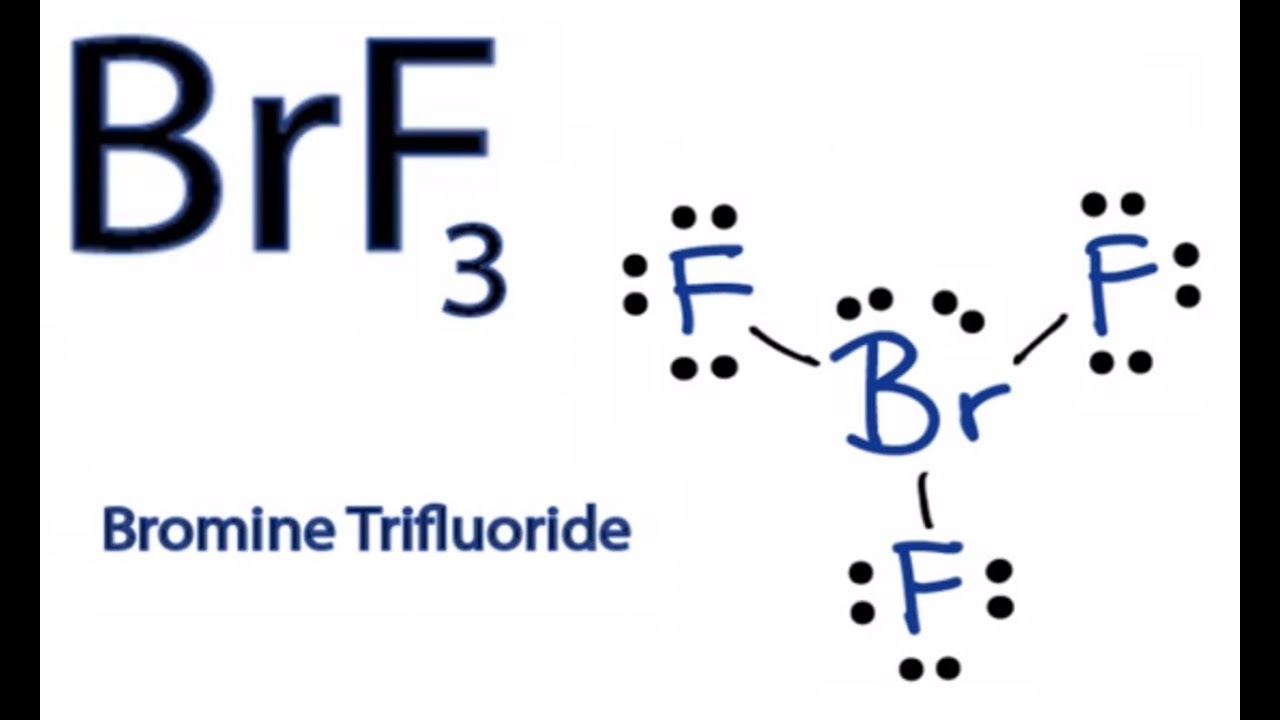
How To Draw The Lewis Dot Structure For Brf3 Boron Trifluoride Youtube
What Is The Hybridization Of Brf3 Quora
Hybridization Of Brf3 Hybridization Of Br In Bromine Trifluoride

Brf3 Lewis Structure Draw The Bromine Trifluoride Dot Structure Geometry Of Molecules
Brf3 Polarity Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules