Lewis Dot For N2h4
Hydrogen H only needs two valence electrons to have a full outer shell. Total 2 lone pairs and 5 bonded pairs present in N2H4 lewis dot structure.

Hydrazine N2h4 Structure Molecular Mass Properties Uses
A step-by-step explanation of how to draw the N2H4 Lewis Dot Structure HydrizineFor the N2H4 structure use the periodic table to find the total number of.

Lewis dot for n2h4. The lewis structure for n2h2 hhnh shows1. Pkirillov is waiting for your help. Since all the atoms are in either period 1 or 2 this molecule will adhere to the octet rule.
Our tutors have indicated that to solve this problem you will need to apply the Lewis Dot Structures. I also like how you set up your posts with not a lot in each but a lot of posts it makes it look separated and then youre not reading too much all at once. Draw the Lewis structures of N2H4 N2H2 and N2Draw the molecules by placing atoms on the grid and connecting them with bonds.
Lewis Dot of Hydrazine. In the Lewis structure for N 2 H 4. N2H4 is polar in nature and dipole moment of 185 D.
Include all lone pairs of electrons and hydrogen atomsUse the bond enthalpies below to answer the following questions. You can view video lessons to learn Lewis Dot Structures. N2h4 Molecular Geometry And Bond Angles Actual Bond Angle Is Less.
Hydrogen is usually surrounded by 4 electrons in a valid lewis structure. Hydrogen H only needs two valence electrons to have a full outer shell. Posted by Amanda at 1159 AM.
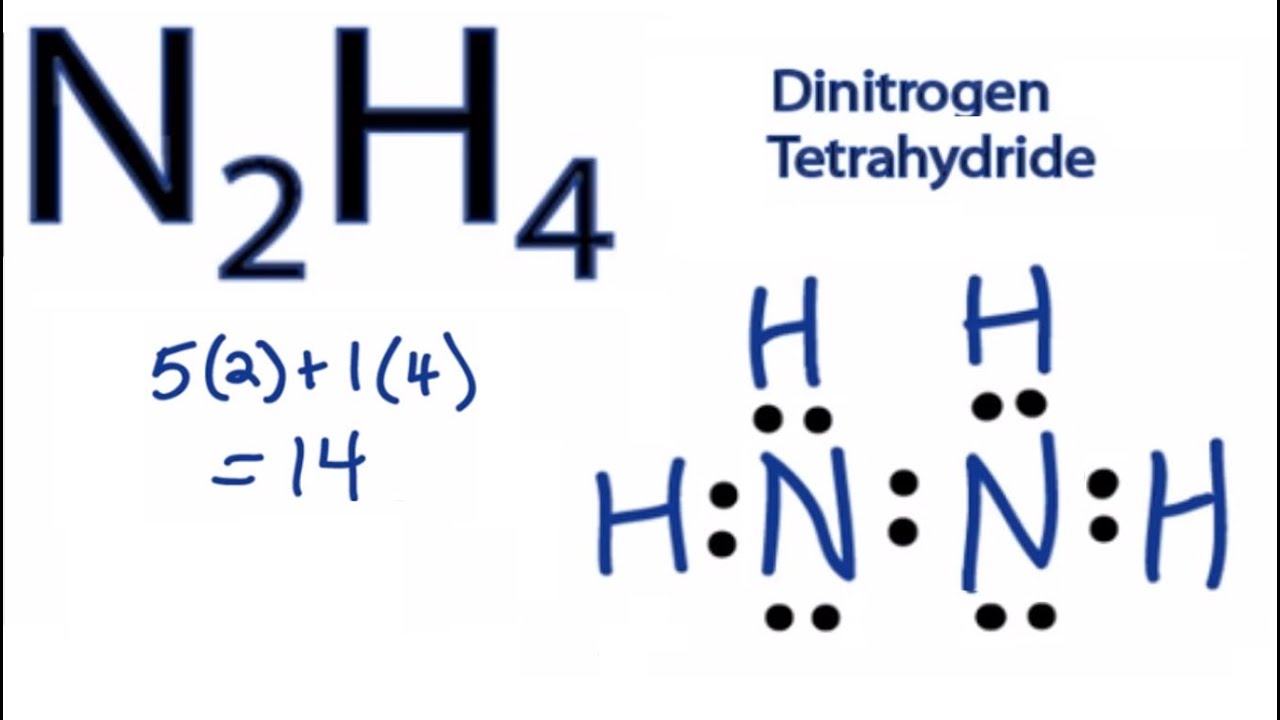
In the Lewis structure for N2H4 there are a total of 14 valence electrons. N2x510 F4x728 Total38 Step2. Lets do the N2H4 Lewis structure.
They follow the duet rule 2 electrons. Nitrogen has five valence electrons. Find valence e- in all atoms.
The exception of course being the hydrogens. You have two Nitrogens. Https Secure Media Collegeboard Org Apc Ap11 Chemistry Q5 Pdf.
Hydrogen H only needs two valence electrons to have a full outer shell. In the N 2 H 4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside. N2 Lewis Structure How To Draw The Lewis Structure For N2.
N2H4 is straightforward with no double or triple bonds. Find octet e- for each atom and add them together. The Lewis Structure for N2H4 with each pair of electron bonds circled in red.
Draw the Lewis dot structure of N2H4. The total valence electron available for the Hydrazine N2H4 lewis structure is 14. Well determine the N2H4 molecular geometry with respect to the Nitrogen on the right the other Nitrogen atom will have the same shape since they are symmet.
Lewis structure of N 2 F 4. Lewis Diagram Ne Diagram Data Pre. The Lewis Structure Lewis Dot Diagram for N2O41.
Name at least 2 ways. Step method to draw lewis structure tetrafluorohydrazine. Forms a colorless gas and has an ammonia like smell.
In the Lewis structure for N2H4 there are a total of 14 valence electrons. Lewis Dot Diagram For N2h4 Written By JupiterZ Thursday December 20 2018 Add Comment Edit. Draw the Lewis structure for N2H4.
Based on the Lewis electron-dot diagrams of n2 and n2h4 compare the length of the nitrogen-to-nitrogen bond in n2 with the length of the nitrogen-to-nitrogen bond in n2h4. What is the hybrid. 70 More Lewis Dot Structures.
Add your answer and earn points. What is the hybrid state of each nitrogen and what nitrogen orbital is involved in forming the double bond. In the N2H4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside.
The formal charge on nitrogen in N2H4 is zero. The Lewis Structure for N2H4 with each pair of electron bonds circled in red. A lewis structure also called lewis dot formulas lewis dot structures or electron dot structures are pictorial diagrams that represent the bonding between atoms in a compound and the placement of electrons.
N 2 H 4 is straightforward with no double or triple bonds. Lets do the N2H4 Lewis structure. The two atomic orbitals AOs involved in the formation of a sigma bond between two hydrogen atoms in the molecule H2 are Draw the.
In the N2H4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside. Draw the Lewis dot structure of N2H2. The hybridization of each nitrogen in the N2H4 molecule is Sp 3.
N2h4 Lewis Structure How To Draw The Lewis Structure For N2h4 Youtube
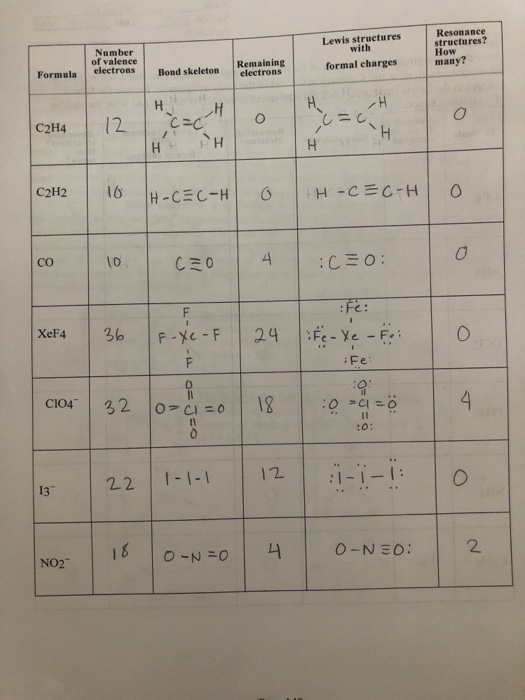
Lewis Structures Formula Number Of Valence Bond Chegg Com

Draw The Molecules Include All Lone Pairs Of Electrons N2h2 N2h4 C2h2 C2h4 H3coch3 Study Com

N2h2 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Https Www Acpsd Net Cms Lib Sc02209457 Centricity Domain 2211 Bonding 20lewis 20structures 20and 20molecular 20shapes 20key Pdf
What Is The Lewis Dot Structure For N2h4 How Is It Made Quora
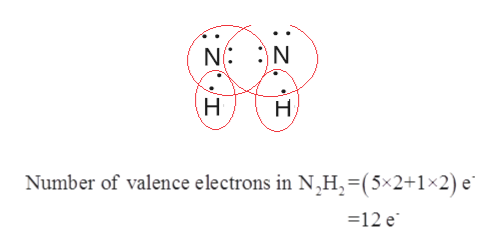
Answered Draw All The Lewis Structures For N2h2 Bartleby

Part D Draw The Lewis Structures Of N2h4 Clutch Prep
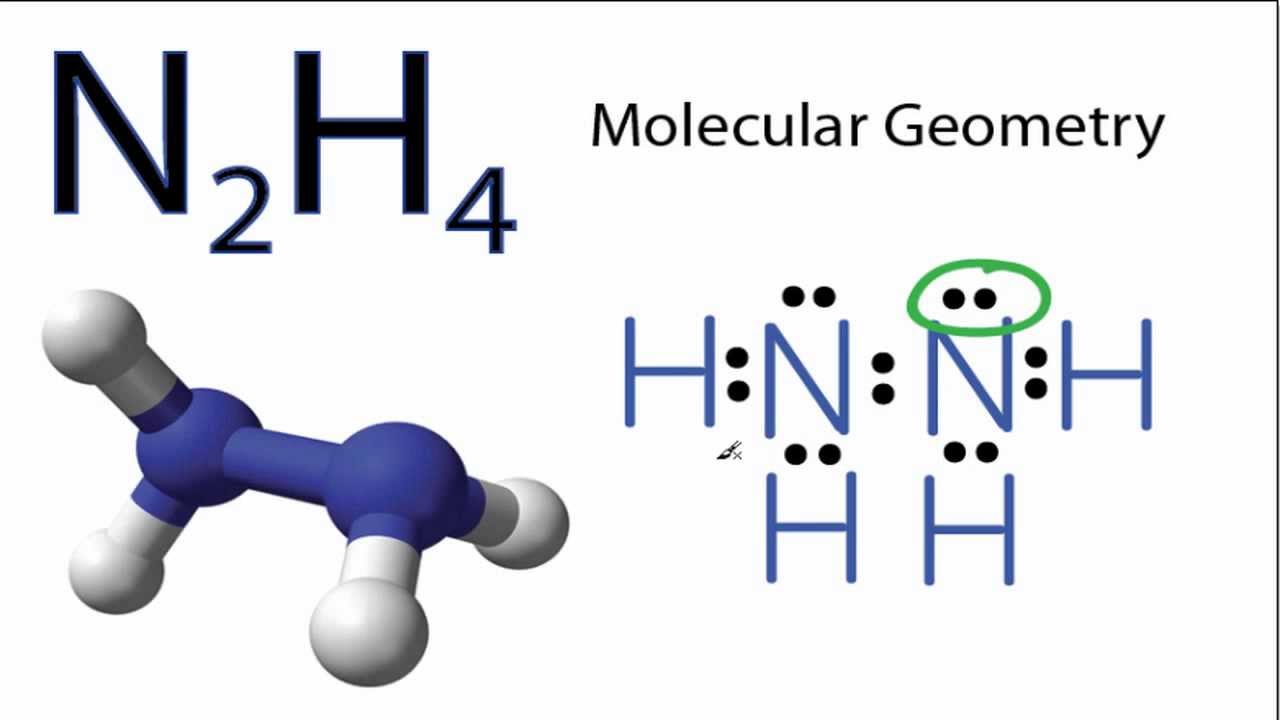
N2h4 Molecular Geometry And Bond Angles Actual Bond Angle Is Less Than 109 5 Degrees Youtube
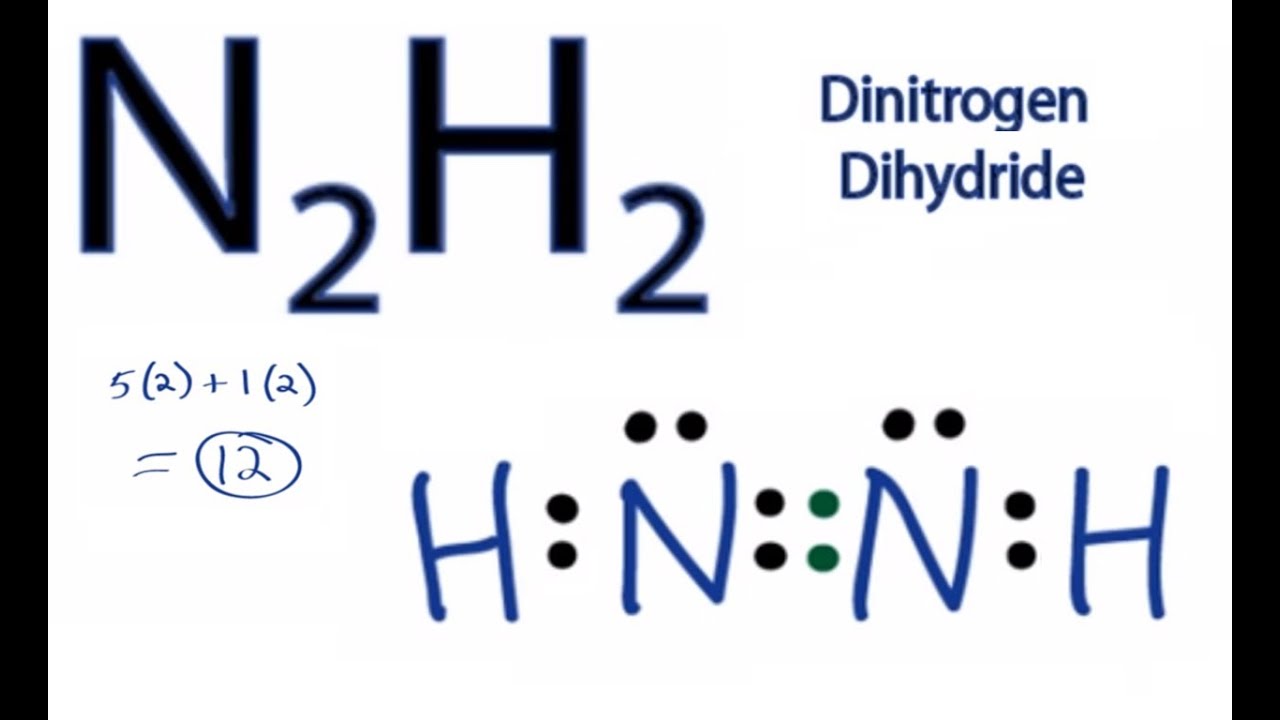
N2h2 Lewis Structure How To Draw The Dot Structure For N2h4 Chemical Bonding

What Is The Lewis Structure For N2h4 Study Com
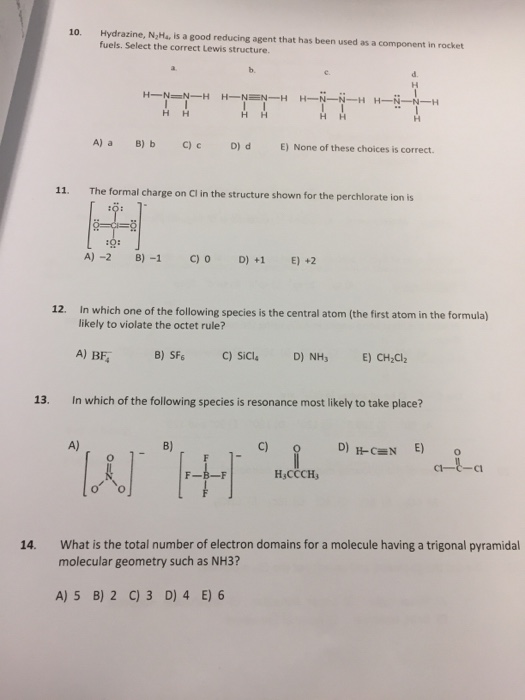
Hydrazine N 2h 4 Is A Good Reducing Agent That Has Chegg Com

Why Doesn T N2h4 Form A Dative Bond Quora

Hydrazine N2h4 Hydrazine Polar Molecule

Hydrazine N2h4 Lewis Structure
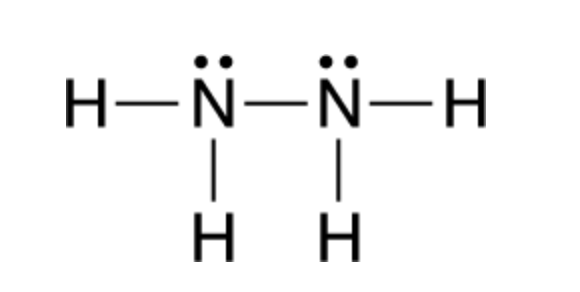
Answer The Following Questions About N2 And N2h4 Chegg Com
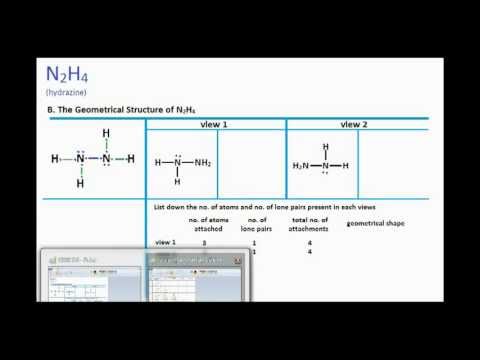
N2h4 Lewis Structure And Molecular Geometry Youtube