Xef4 Lewis Structure Resonance
While in resonance structure B the xenon has a charge of 4 and each oxygen has a charge of -1. XeF 4 is d 2 sp 3 hybridized and contains 2 lone pair and 4 bonding pairs of valence.

Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules
The main purpose of these Lewis structures is to show that the octet rule is quite important during chemical bonding.
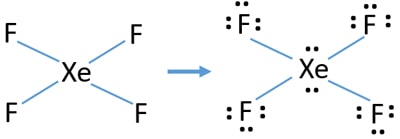
Xef4 lewis structure resonance. It helps in knowing the electronic structure primarily. 4 Xe-F single bonds use up 8 of these electrons and 3 non-bonding pairs of electrons complete each octet on the F atoms for a total of 24 more ve. That will make a total of 12 electrons or 6 pairs.
Subtract step 1 total from step 2. Step 2 The next step asks us to distribute the valence electrons in the molecule all around the central atom. 3 a CHCl3 b NH4 c H2CO 6.
When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. Does formaldehyde have resonance structures. Bromine gets 12 electrons in order to make 5 bonds with surrounding atoms.
FNO2 resonance structure molecular polarity direction hybrid used. Of XeF4 step by step. Drawing Lewis structure of XeF4.
XeF2 Lewis Structure. Xe does not follow the octet rule. Lewis Structure 3-D Sketch Are There Resonance Structures SN Valence e- Electron Count.
The Lewis structure for XeF 4 requires you to place more than 8 valence electrons on Xe. Resonance structures of equal and unequal stability. Find valence e- for all atoms.
This Lewis dot structure is a pictorial representation of valence electrons around individual atoms in a molecule along with the bond it forms. Xe has 8 electrons in its outer valence shell. Xenon Xe can have more than 8 valence electrons in your Lewis structure.
Xenon tetrafluoride XeF4 is a square planar non-polar molecule. Xenon is an inert gas element. In resonance structure A the xenon and oxygens are neutral.
We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more no less. HOCN resonance structure molecular polarity direction hybrid used. So the resonance structures tell us that the xenon-oxygen bonds in X e O X 4 are some mix of single and double bond character.
70 More Lewis Dot Structures. We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more no less. We use dots to represent outer shell electrons and lines to represent the bond type.
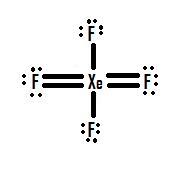
Bonding e- groups. The X e O bonds in X e O X 4 are very weak. The Xenon atom has 4 bonding pairs of electrons and 2 lone non-bonding pairs of electro.
In the XeF 4 Lewis structure Xe is the least electronegative and goes at the center of the structure. Xe has 8 ve 4 F atoms have a total of 28 so there are 36 total ve in the molecule. XeF4 Lewis Structure Now that we know the valence electrons of Xenon Tetrafluoride it will be easier for you to draw its Lewis structure.
Thus you will have 4 Xe-F bonds and 2 lone pairs. Step 1 We need to count the valence electrons of the xenon tetrafluoride molecule with the help of a periodic table. IF4 1- resonance structure molecular polarity direction.
The Lewis structure for XeF4 has a total of 36 valence electrons. Gives you bonding e-. Drawing the Lewis Structure for XeF 4.
Draw the Lewis structure for i CO ii XeF4 iii PCl3 By signing up youll get thousands of step-by-step solutions to your homework. Write Lewis structures that obey the octet rule for each of the following. Lone pairs will take up more space than Xe-F bonds so the lone pairs.
The Lewis structure for XeF4 has a total of 36 valence electrons. Write Lewis structures for the following molecules or ions which have central atoms that do not obey. Lewis Structure also known as electron dot structure is an essential model of chemical bonding where we use the valence electron concept to schematically sketch a two-dimensional figure of a given molecule.
4 F atoms will add 4 electrons to make 4 Xe-F bonds. In XeF4 Xenon tetrafluoride lewis structure there are four sigma bonds and two lone pairs Each fluorine atom has three lone pairs. XeF4 resonance structure molecular polarity direction hybrid used.
Show ALL resonance structures where. NCCN resonance structure molecular polarity direction hybrid used. PBr5 SCl4 XeF4.
It will hold more than 8 electrons. Find octet e- for each atom and add them together. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell.
Step method to draw lewis structure for XeF4 This molecules is an example of expanded octet Step 1. Xenon having valence electrons in the 4th energy level will also have access to the 4d sublevel thus allowing for more than 8 electrons.

Gen Chem 2 3 Flashcards Quizlet
13 12 Exceptions To The Octet Rule Chemistry Libretexts

Xef4 Lewis Structure And Molecular Geometry Youtube

In The Best Lewis Structure For Xef4 What Is The Formal Charge On The F Atom A 1 B 0 C 1 D 2 Study Com
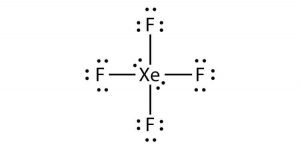
Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
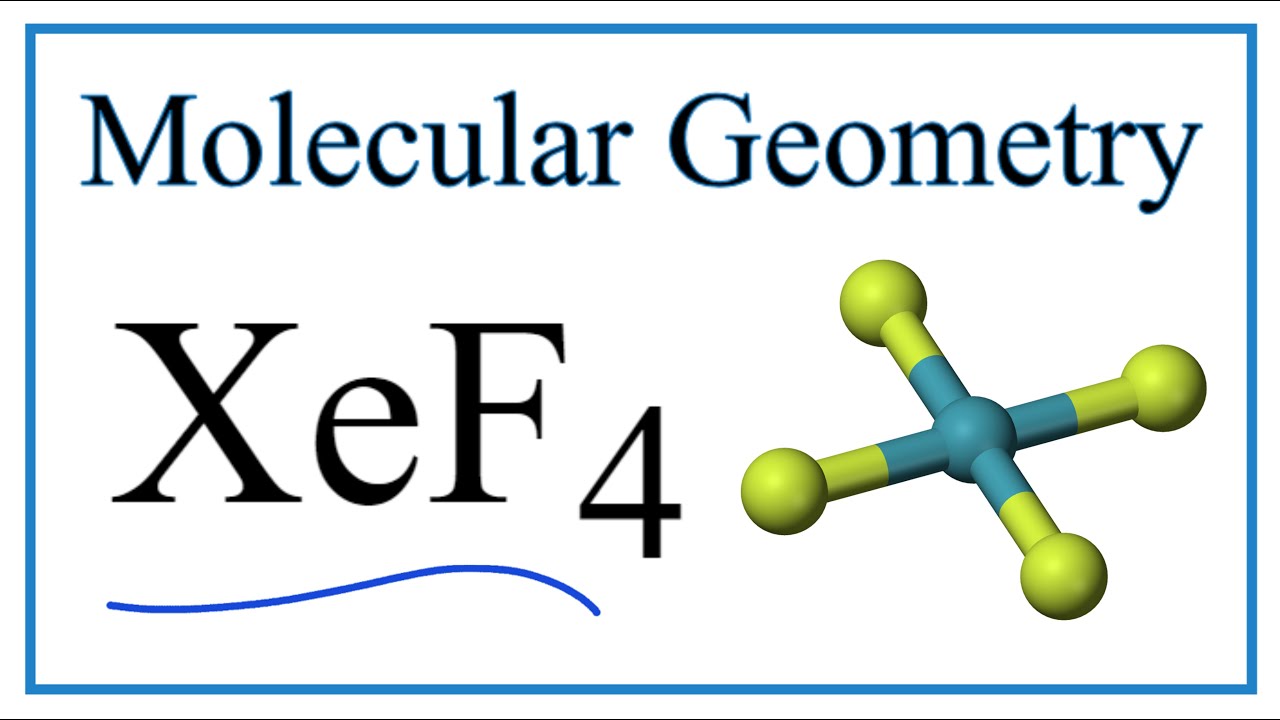
Xef4 Molecular Geometry Bond Angles Electron Geometry Youtube
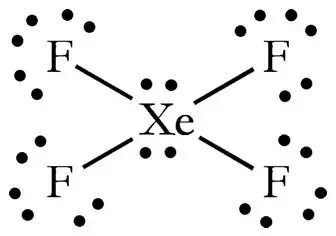
How Many Pairs Of Electrons Are There In The Lewis Structure Of Xef4 Quora
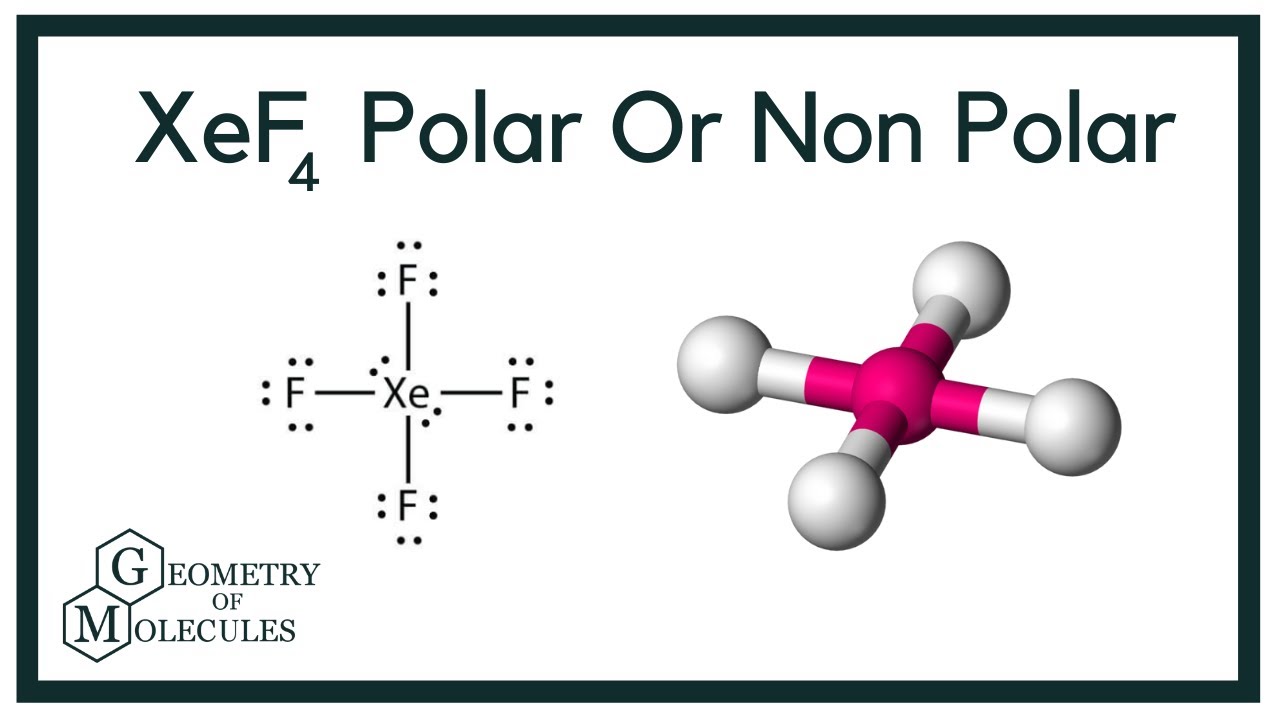
Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules
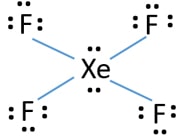
Xef4 Xenon Tetrafluoride Lewis Structure
Chem 101 Octet Rule Violations

How To Calculate The Formal Charges For Xef4 Xenon Tetrafluoride Youtube

Xef6 Lewis Structure How To Draw The Lewis Structure For Xef6 Youtube

Why Does The Lewis Structure Of Xef4 Not F Clutch Prep

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Xef4 Xenon Tetrafluoride Lewis Structure

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 Youtube
Vsepr Shape Of Tef4 Biochemhelp
