Sif4 Is Lewis Acid
Its boiling point is only 4 C above its melting point. Under prolonged exposure to heat the containers may rupture violently and rocket.
Which Are Of The Following Are Lewis Acids Cheek Chegg Com
Heres how SiF4 acts like a Lewis acid.
Sif4 is lewis acid. Why does SiF4 act as a lewis acid. Most compounds which are Lewis acids require an activation step before production of the adduct with Lewis base. Hence order of Lewis acid.
The H ion would be accepted by another compound base to form a bond. From chemwikiucdavisedu SiF_4 can accept electron pairs and expand its octet to 12. It is a tetrahedral molecule.
SiF4 can accept electron. Heres how SiF4 acts like a Lewis acid. Please explain how SiF4 is a Lewis Acid.
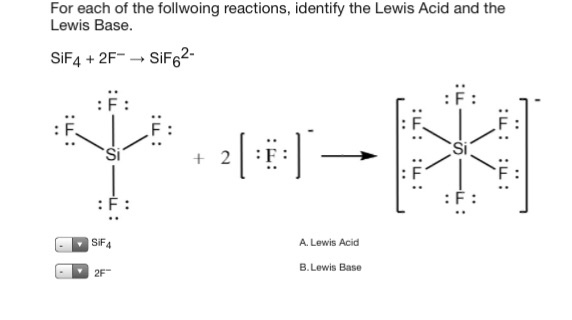
It was first synthesized by John Davy in 1812. Essentially H can be a Lewis or Bronsted acid it just depends how. F- to give SiF6 2- ion.
This Question has Been Answered. Our videos will help you understand concepts solve your homework and do great on your exams. 1 Answer Ernest Z.
Vapor is heavier than air. An example of such a Lewis acid would be BR 3 where R can be a halide or an organic substituent. Depending on the nature of ceX- the pentacoordinated ceSiF4X- species may either break down under liberation of either ceF- mathrmS_NSi substitution or ceX-.
On the other hand such process is not possible for compounds formed by 2nd period elements. Watch 1000 concepts tricky questions explained. Chemistry 3 Months Ago 18 Views.
How would it be able to accept an electron pair. Silicon is larger than carbon and better able to stabilise 4e3c bonds due to its lower electronegativity. Our videos prepare you to succeed in your college classes.
Jan 4 2016 SiF_4 can act as a Lewis acid because Si can expand its octet. SiF4 2F- ÃÂÂ SiF62- SF4 F- ÃÂÂ SF5- Its taking that negative charge electrons from the F-Be careful not to confuses these with the Bronsted-Lowery acidsbases in which. Hence they can not act as Lewis Acid e.
In many cases the Lewis acid can bind two Lewis base a popular example being the formation of the hexafluorosilicate. Please correct my logic. SARL K FOOD COMPANY.
Loading DoubtNut Solution for you. I am calculating 32 Valence Electrons. When drawing out the Lewis Dot Structure 32 Electrons satisfies the octet rule for all 5 atoms in the molecule.
Most compounds which are Lewis acids require an activation step before production of the adduct with Lewis base. Let us help you simplify your studying. Organic Chemistry Acids and Bases Main Characterstics or LewisBronsted Definition.
329 K views 16 K people like. To begin with SiF₄ does fulfill the definition of Lewis acid because it can accept electron pairs those from say fluoride anions in a vacant orbital low lying energetically available 4 p orbitals. Can H be a Lewis base.
This term was classically used to describe chemical species with a trigonal planar structure and an empty p-orbital. Apne doubts clear karein ab Whatsapp par bhi. This colorless compound is notable for having a narrow liquid range.
So it not act as lewis acid. SiF4 can act as a Lewis acid because Si can expand its octet. Now in case of SiF4 molecule 3d- orbitals are empty hence it can accept electron pair from Lewis base eg.
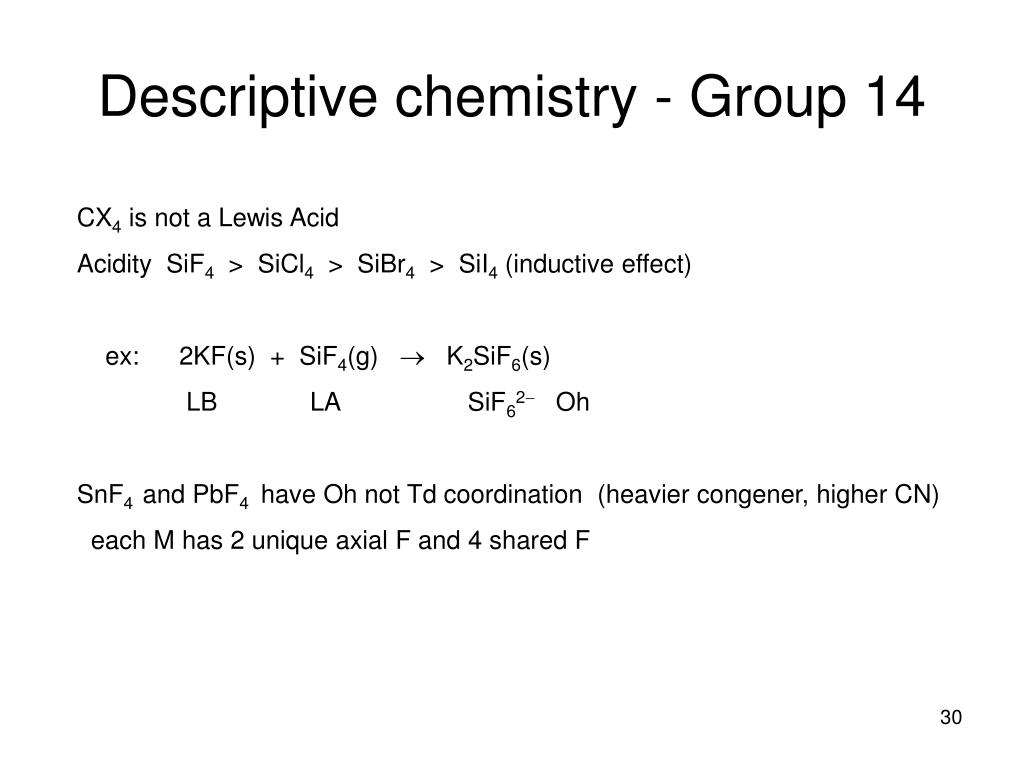
SiF4 SiCl4 SiBr4 SiI4 In case of silicone halides inductive effect dominate over back bonding hence Lewis acid character decided by inductive effect. Let us help you simplify your studying. If you are having trouble with Chemistry Organic Physics Calculus or Statistics we got your back.
Silicon tetrafluoride appears as a colorless nonflammable corrosive and toxic gas with a pungent odor similar to that of hydrochloric acid. Why is sicl4 not a Lewis acid. A base is a species that accepts protons H while an acid is a species that dontates protons H.
Cyanide is isolectronic with CO and while not as pi-. BF3 AIF3 SiF4 and PF5 all contain electron deficient inner atoms. CCl4 can not keep more than 8 electrons in its outermost orbit due to the absence of d-orbitals.
Similarly popular cases are the aluminum trihalides that are visible widely as Lewis acids. Why is SIF_4 a stronger Lewis acid a s compared to SiCl_4. Lewis acid can accept a pair of electron from Lewis base.
Lewis Acids are the chemical species which have empty orbitals and are able to accept electron pairs from Lewis bases. 5 1 Ratings Solved. SiF4 2F- SiF62- SF4 F- SF5- Its taking that negative charge electrons from the F-Be careful not to confuses these with the Bronsted-Lowery acids.
Thus 4e3c bonds are sufficiently accessable and stable for silicon in ceSiF4 to act as a Lewis acid. A Lewis acid is an electron-pair acceptor. Search More info Main menu.
Silicon tetrafluoride or tetrafluorosilane is the chemical compound with the formula SiF4. SiF4 2 F SiF62. Very toxic by inhalation.

Which Are Of The Following Are Lewis Acids Cheek Chegg Com

For Sif4 Draw The Lewis Structure Predict The Shape And Determine If The Molecule Is Polar Or Nonpolar Study Com
For Each Of The Following Reactions Identify The Chegg Com
Part B For The Following Reaction Identify The Lewis Chegg Com
Why Does Sif4 Act As A Lewis Acid Example

A For Sif4 2f Sif6 Identify The Lewis Acid And Chegg Com

Is Sif4 A Lewis Base Or Lewis Acid
Solved The Uorides Bf3 Aif3 Sif4 And Pf5 Are Lewis Acids The All Form Very Stable Uoroanions When Treated With Lithium Uoride In Contrast The Course Hero

Incorrect Order Of Lewis Acid Character Youtube

What Is The Lewis Structure Of Sif4 And How Does It Compare To That Of Nitrogen Quora
Silicon Tetrafluoride Wikiwand
What Is The Acidic Order Of Sif4 Sicl4 And Sibr4 Quora

Which Of The Following Is Not A Lewis Acid A Sif4 B C2h4 C Bf3 D F
Ppt Ch4 Acids And Bases Powerpoint Presentation Free Download Id 4263579

Is Sif4 A Lewis Base Or Lewis Acid

Silicon Tetrafluoride Sif4 Lewis Dot Structure Youtube

Solution Which Of The Following Is A Lew Chemistry