Lewis Dot Structure For C2h2f2
Select all the correct answers. The valency of carbon is four so it likes to have exactly four bonds so if the each carbon atom is bonded to two non - carbon atoms the.

Lewis Structure Teaching Chemistry Chemistry Classroom Chemistry Education
Ne 3s23p3 Lewis Structure Shows only the valence electrons P.
Lewis dot structure for c2h2f2. Draw the Electron dot structure. For the molecule we expect the carbons to be the central atoms in this molecule since carbon tends to be the central atom in its compounds. I know you draw the lewis structure but when I drew it I thought.
Tell me about the best Lewis structure. Draw a Lewis structure for ozone. Draw the Lewis structure for the molecule.
For this C2H2Br2 Lewis structure we really should call it 12-Dibromoethene. Explain why one of the three structures for CHCl is nonpolar and the other two are mol ecular dipoles. I know how to draw the lewis structure but Im not sure how to find the molecular geometry.
CO 3 2 4. Lewis Symbols or Dot Diagrams or Lewis Dot Diagrams Phosphorus Electron configuration. Using a structure estimation method based on molecular connectivity indices 1 the Koc of 12-difluoroethane can be estimated to be 40 SRC.
What is THE LEWIS DOT STRUCTURE FOR C2H2F2. Use VSEPR theory to predict the molecular geometry around either carbon atom in acetylene C2H2. Draw the Lewis dot structure for each of the following polyatomic ions.
Remember that Hydrogen H atoms always go on the outside of a Lewis Structure. PO 4 3 b. How do you figure out the answer is linear.
Hydrogen is usually surrounded by 4 electrons in a valid Lewis structure. What is the molecular geometry of trans-difluroethylene. Alternatively a dot method can be used to draw the lewis structure of C 2 F 2.
The -ene means we have the double bond here between the Carbons and the Bromines are on the first and second Carbon in this structure. Put two canbon atoms in the center side by sidePut one fluorine on each carbon atom. The Lewis structure of the compound involves the representation of symbols of the elements surrounded by dots which indicates the electrons taking part in the bond formation as well as the non-bonding electrons.
What is THE LEWIS DOT STRUCTURE FOR C2H2F2. The Answer is linear. NH 4 c.
A step-by-step explanation of how to draw the CH2F2 Lewis Dot Structure DifluoromethaneFor the CH2F2 structure use the periodic table to find the total nu. In the Lewis structure for C 2 H 2 Cl 2 there are a total of 20 valence electrons. Draw the Lewis dot structures for each of the following molecules.
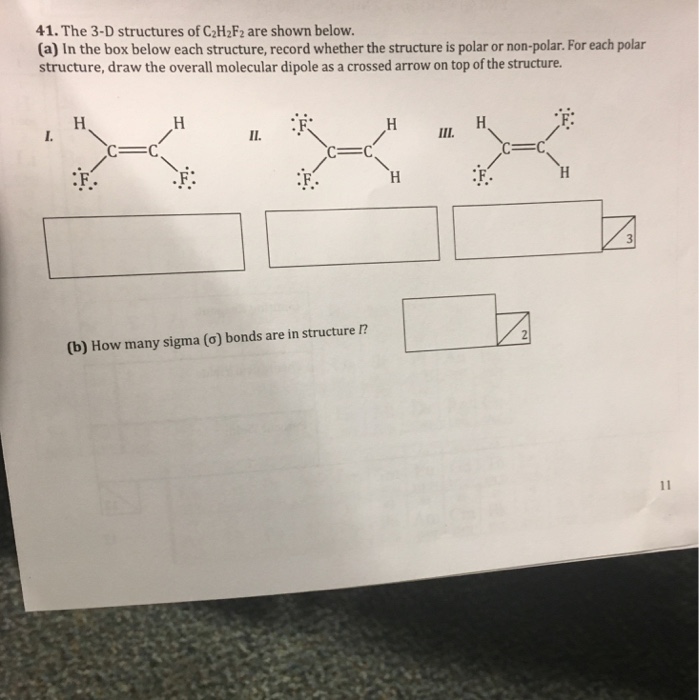
It looks like a lewis dot structure for C2H2F2. Draw the other two structures and indicate whether each one is nonpolar or a dipole. Were being asked to draw the 3 Lewis structures for C 2 H 2 Cl 2 and indicate whether each is non-polar or polar.
A single bond in a Lewis structure represents 2 electrons. Draw three Lewis structures for compounds with the formula C 2 H 2 F 2. Calculate the total number of valence electrons present.
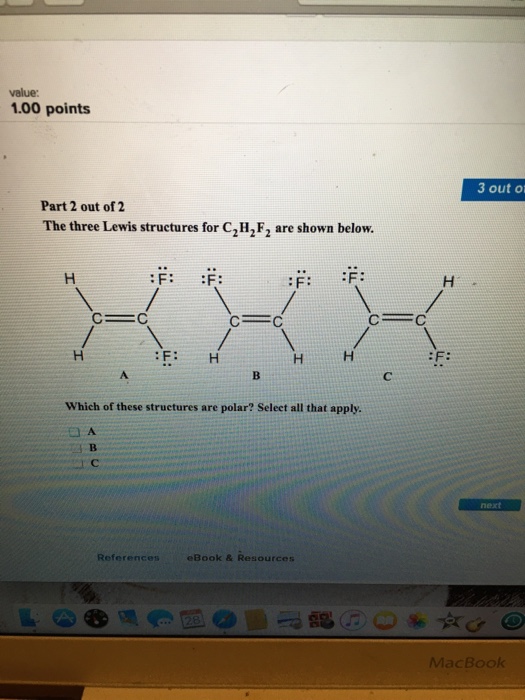
Indicate which of the compound s are polar. To do so we first need to do the following steps. NO 3 d.
Tioderbo c2h2f2 lewis dot structure c2h2f2 lewis dot structure - Hcg failure Tell me about the atomic charges dipole moment bond lengths angles bond orders molecular orbital energies or total energy. The Lewis dot structure would be Cr with one dot over it. I think its a tetrahedral.
H 2 S c. According to a classification scheme 2 this estimated Koc value suggests that 12-difluoroethane is expected to have very high mobility in soil. Note that Hydrogen only needs two valence electrons to have a full outer shell.
Carbon is the least electonegative atom so it goes at the center of the C 2 H 2 Cl 2 Lewis structure. There are three acceptable Lewis structures for CaH Cl2 and you have drawn one of them on the report form. So thats the Lewis structure for C2H2Br2.
Hydrogen is usually surrounded by 4 electrons in a valid Lewis structure. Calculate the total valence electrons in the molecule. For the following molecules or ions where the central atom is underlined.
5 Electronic Nirvana The octet rule - 8 valence electrons. CH 2 Br 2 d. If both Bromines were on the first Carbon here we would call it 11-Dibromoethene.
Determine the central atom in this molecule. A single bond in a Lewis structure represents 2 electrons.
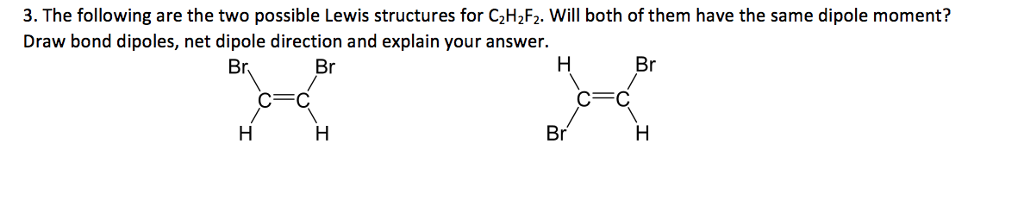
3 The Following Are The Two Possible Lewis Chegg Com
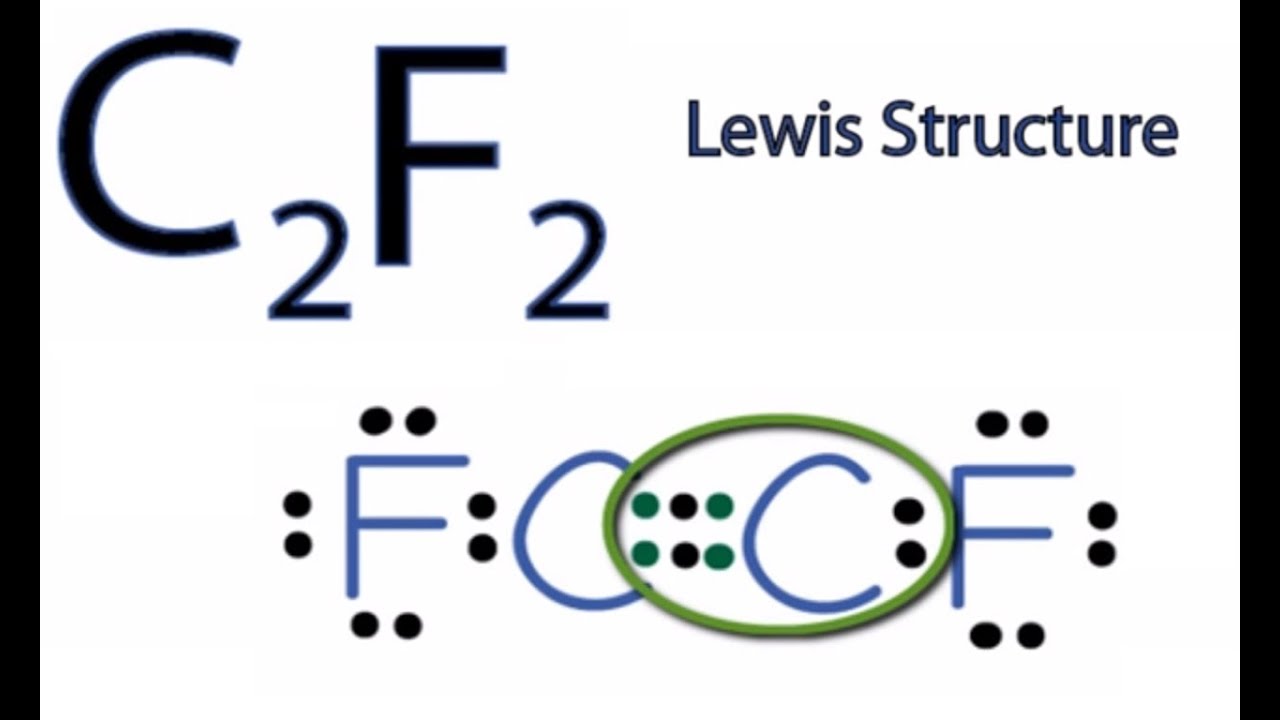
C2f2 Lewis Structure How To Draw The Lewis Structure For C2f2 Youtube
The 3 D Structures Of C 2h 2f 2 Are Shown Below A Chegg Com
The Three Lewis Structures For C 2h 2f 2 Are Shown Chegg Com

Chf3 Lewis Structure How To Draw The Lewis Structure For Chf3 Youtube

Covalent Problem C2h2f2 Youtube

C2h2br2 Lewis Structure How To Draw The Lewis Structure For C2h2br2 Youtube
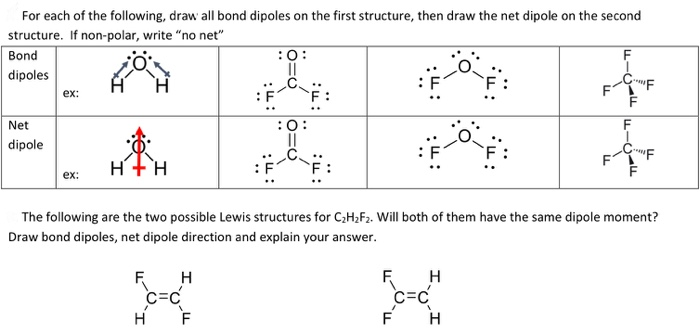
For Each Of The Following Draw All Bond Dipoles On Chegg Com
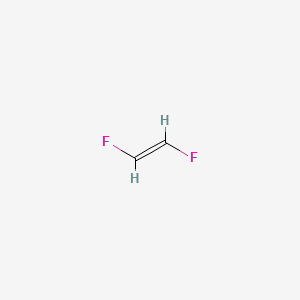
1 2 Difluoroethylene C2h2f2 Pubchem

C2h2cl2 Lewis Structure How To Draw The Lewis Structure For C2h2cl2 Youtube
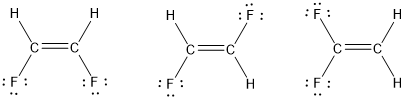
Solved Draw Three Lewis Structures For Compounds With The Formula Chegg Com

1 Chemical Bond Ionic Bond 4 Types Of Bonds Covalent Bond Ppt Download

Chm 101 R P For Exam 2 2010 Big Chm101 02

1 Determine The Number Of Valence Electrons Then Chegg Com

C2f2 Lewis Structure How To Draw The Lewis Structure For C2f2 Youtube