Lewis Structure Of Sf6
What is the hybridization on the Br atom. Given molecule is SF6 S F 6 that is sulfur hexafluoride.
Lewis Structure Sulfur Hexafluoride Lewis Pair Vsepr Theory Sulfur Tetrafluoride Png 800x773px Watercolor Cartoon Flower Frame
SF4 Lewis Structure Molecular Geometry Hybridization and MO Diagram.

Lewis structure of sf6. Upon inhalation of sulfur hexafluoride SF6 the gas is distributed throughout the lungs. Sulfur hexafluoride or SF6 is. SF4 or sulfur tetrafluoride is a compound that has a distinct odor of sulfur or rotten eggs.
Sulfur Hexafluoride is a contrast agent composed of an inorganic fluorinated inert gas comprised of six fluoride atoms bound to one sulfur atom with potential diagnostic activity upon imaging. SF4s boiling and melting points are -38. 5 rows SF6 Molecular Geometry Lewis Structure Shape and Polarity.
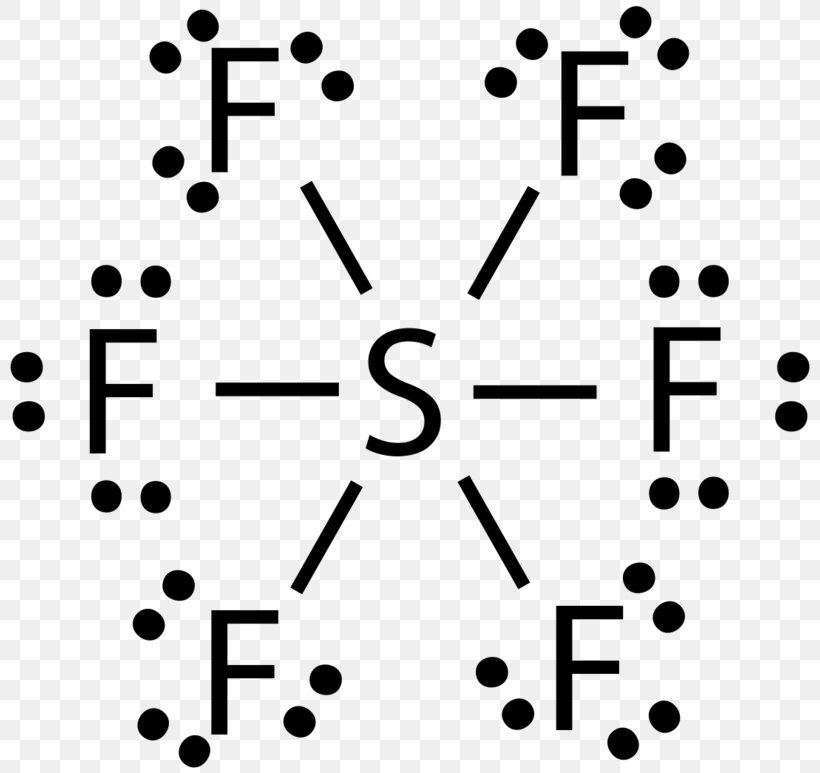
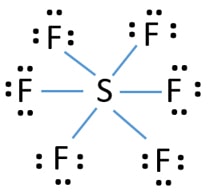
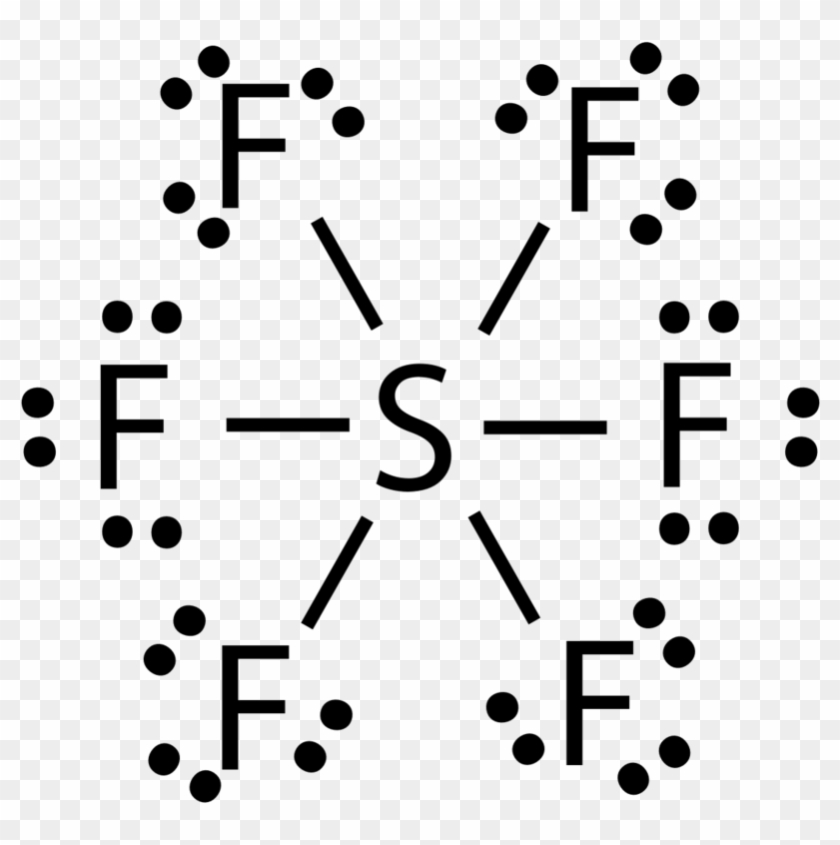
There are no lone pairs on sulfur atom and three lone pairs on each fluorine atom. The central atom here is sulfur bonded with 6 fluorine atoms. Lewis dot structure of SF 6.
What is the hybridization on the Br atom. What is the hybridization on the S atom. SF 6 Sulfur hexafluoride molecule contains one sulfur atom and six fluorine atoms.
Lewis structures a lewis structure is a representation of covalent bonding where shared electron pairs are shown as lines and lone electron pairs are shown as dots. Lewis structure of SF 6 is given below. Use information from step 4 and 5 to draw the lewis structure.
6 rows There are a total of 48 valence electrons in the Lewis structure for SF6. It is inorganic colorless odorless non-flammable and non-toxic. Central atom sulfur and each of the neighbouring atoms fluorine has six and seven valence electrons.
Sulfur Hexafluoride SF 6 Lewis and Three-Dimensional Structures Octahedral 3-Dimensional View of SF 6 Chemistry Home Dr. SF 6 Sulfur hexafluoride Lewis Structure. Draw the lewis structure for SF6 and then answer the following questions.
Put sulfur in the center and five fluorine atoms on the sides. Thus the sf6 electron geometry is considered to be octahedral. Draw the Lewis structure for BrF5.
Calculate the total valence electrons in the molecule. Draw the Lewis structure for SF6. D hybridization of sulfur and possible bond angles.
To do so we first need to do the following steps. Alternatively a dot method can be used to draw the lewis structure. What is the hybridization on the Br atom.
Calculate the total number of valence electrons present. 60 Draw the Lewis structure for SF6. SF 6 has an octahedral geometry consisting of six fluorine atoms attached to a central sulfur atom.
Determine the central atom in this molecule. Draw the Lewis structure for SF6. The molecular geometry of sf6 is octahedral.
I quickly take you through how to draw the Lewis Structure of SF6 Sulfur HexaFluoride. Determine the hybridization at each of the 2 labeled carbons. B electron domain geometry of SF6.
We are asked how many electrons does the central atom S shares in the Lewis structure for SF 6. I also go over the hybridization shape and bond angles. This problem has been solved.
Draw the Lewis structure for the molecule. In SF 6 lewis structure each fluorine atom has made single bonds with center sulfur atom. C molecular geometry of SF6.
Consider the molecule below. There are three lone pairs on each fluorine atom. What is the hybridization on the S atom.
The molecular weight of this compound is calculated to be 1086 gmol. Draw the Lewis structure for BrCl3. It has a molecular geometry of the formula AX4E.
The hybridization of SF6 is sp3d2. Note that Sulfur S. Sulfur Tetrafluoride has 34 valence electrons out of which it forms four covalent bonds and one lone pair of electrons on the central atom in its Lewis structure.
Count the shared electrons. It forms a see-saw shape and has a trigonal bipyramidal molecular geometry. 107 61 Draw the Lewis structure for BrF5.
Sulfur hexafluoride SF 6 or sulphur hexafluoride British spelling is an extremely potent and persistent greenhouse gas that is primarily utilized as an electrical insulator and arc suppressant. Lewis dot structure has 6 sigma bonds and rests lone pairs on fluorine. What is the hybridization on the S atom.
SF6 is a colorless and odorless gas that is non-combustible and non-flammable in nature. This compound is generally identified as being a colorless gas. Sundin Home sundinuwplattedu.
Sf6 Sulfur Hexafluoride Lewis Structure

Sf6 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
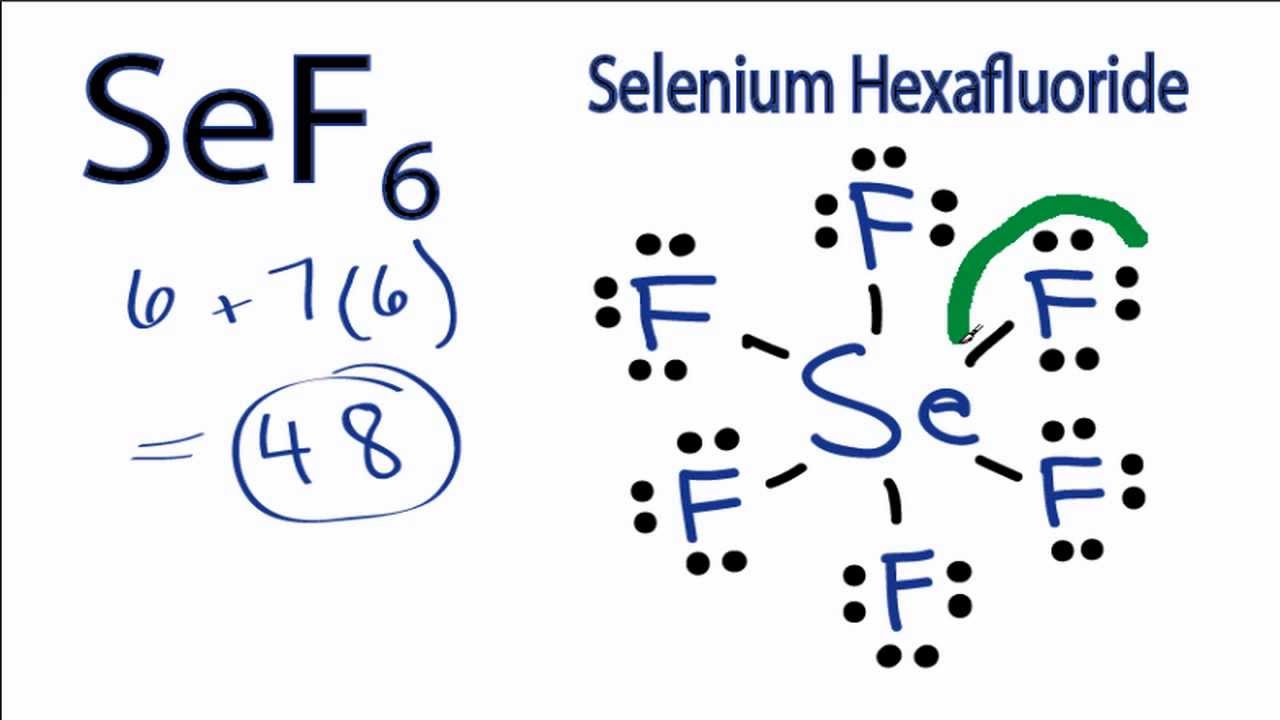
Sef6 Lewis Structure How To Draw The Lewis Structure For Selenium Hexafluoride Youtube
Molecular Geometry Ck 12 Foundation

Sf6 Lewis Structure How To Draw The Lewis Structure For Sf6 Youtube

In The Lewis Structure For Sf6 The Centr Clutch Prep

Hybridization Of Of2 Oxygen Difluoride In 2021 Molecules Oxygen Things To Come

Sf4 Lewis Structure How To Draw The Lewis Structure For Sf4 Youtube
Drawn Molecule Sf6 Sf6 Lewis Dot Structure Free Transparent Png Clipart Images Download

Sf6 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Sf6 Lewis And 3 D Structures Dr Sundin Uw Platteville

Draw The Lewis Structure Give The Name And Predict The Vsepr Geometry Of A H2o B Nh3 C Sf6 Study Com
Lewis Structure Of Sf6 Biochemhelp
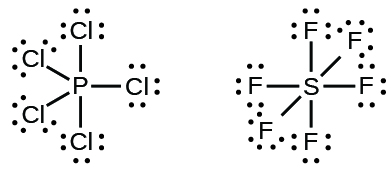
4 2 Lewis Structures Chemistry Libretexts

A Sf6 B N2o4 Draw Lewis Structures For The Formula Above Include Any Resonance Structures If More Than One Lewis Structure Can Be Drawn Use Formal Charges To Decide On The Most
Solved Draw Lewis Dot Electron Structure For Sf6and Determine The Following Course Hero

Brf3 Lewis Structure Bromine Trifluoride In 2021 Lewis Chemical Formula Dots