C2h4 Lewis Structure Bond Angle
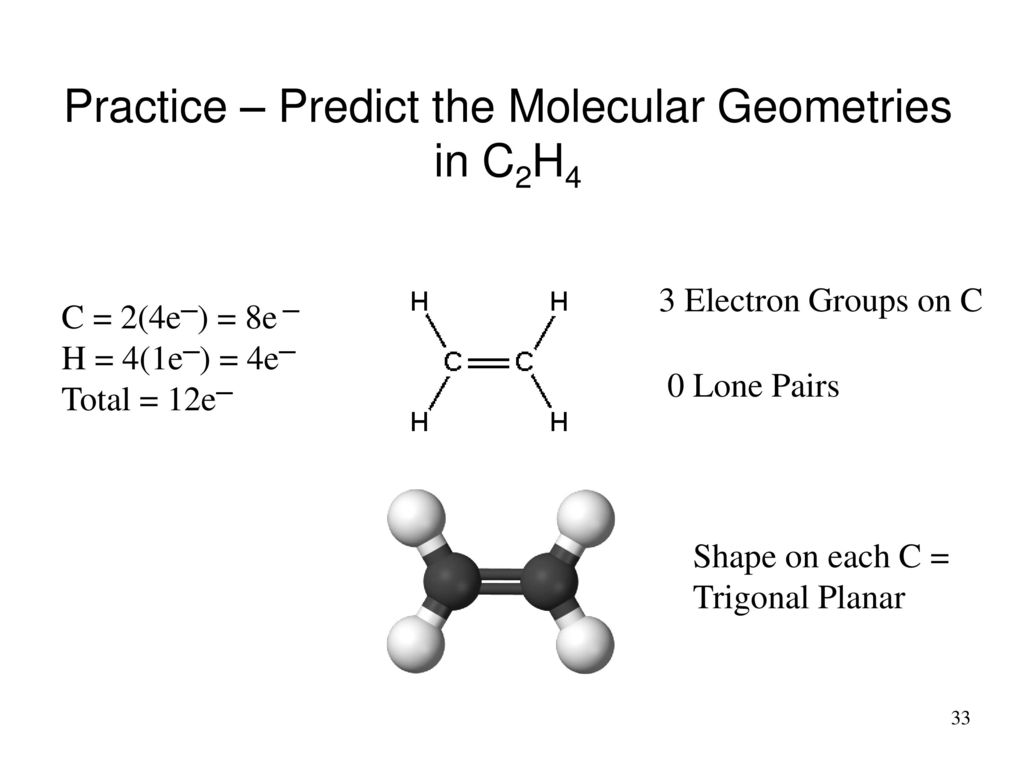
A 180 B About 120 C Ab t 109 5About 1095 D A little larger than 1095 E A little smaller than 1095 Why predicting bond angles is important H-C-H H-C-C C-C-O O-C-O C-O-H bond angle Being able to predict bond angles. The molecular shape is predicted to be trigonal planar around each carbon atom.

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Complete the following table by first drawing a Lewis structure and geometric sketch for each formula.

C2h4 lewis structure bond angle. Accordingly what is the bond angle of c2h2. There are two triangles overlapping each other as we can see in the diagram. According to the VSEPR chart the shape of the ethene molecule is trigonal planar.
Chemical formula Lewis structure Geometric sketch including bond angles CH4 OCl2 NCl3 CO2 CH2O. Read More About Hybridization of Other Chemical Compounds. Ethylene C2H4 has sp2 hybridization and its bond angle is between 1166 to 1217.
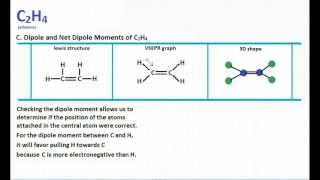
C2H4 molecular geometry is said to be planar in structure while the sp 2 orbitals are placed at a bond angle of 120 o. C2H6 lewis structure. C2H4 Lewiss dot structure is very helpful to find Its molecular geometry because the lewis diagram helps us to determine how many bond pairs and lone pairs a molecule contains.
Ethane is an organic compound with a chemical formula of C2H6. Here A central atom X surrounding atoms and E the lone pairs. The bond angle is around 120 degrees.
It is a chemical formula for Ethylene or Ethene. See the title structure below Flat but I cant see anything from the angle. C2H4 Molecular Geometry And Bond Angles.
The electron dot structure widely known as Lewis Structure is a skeletal diagrammatic representation of a molecule taking into account the constituent atoms and the valence shell electrons. To know whether resonance structures can be drawn for C 2 H 4 you should understand the structure of lewis structure of C 2 H 4In the lewis structure of C 2 H 4 there are only four C-H bonds one CC bond and no lone pairs on last shellsThere are only single bond between carbon atom and hydrogen. This is due to the fact that each carbon surrounds a planar triangle.
Each HCH bond angle is around 1175º because the presence of a double bond in between carbon atoms shrinks the angle between the HCH bond from 120º to 1175º. Molecular geometry This is ethane an alkyne double H to H with 2 carbon atoms which means that the relationship between the carbon atoms is double. We can easily find out the molecular geometry of any compound using the given chart.
This compound is one of the simplest hydrocarbons to exist having a single bond between carbon atoms. Is C2H4 trigonal. This is composed of a σ framework and a π-bond.
The molecular geometry of SO2 is bent with a bond angle of 120. SO2 is an AX2E type molecule with 2 surrounding atoms ie oxygen and 1 lone pair of sulfur. This means that the.
CH4 H2O SO2 CO2 NH3 SO3 CH212. C2H4 Molecular Geometry As it can be seen from the Lewis structure above each Carbon atom is bonded to two Hydrogen atoms. What is the C-O-H bond angle in CH 3COOH acetic acid.
The first one has been completed as an example. The molecular geometry of C2H4 is trigonal planar above are the explanation of its Lewis structure. The bond angle is around 120 degrees.
Secondly what does c2h4 look like. According to the VSEPR theory the Hydrogen atoms on both Carbon atoms will repel each other giving rise to a bond angle of 1213. Its slightly soluble in water.
C2H4 Bond Angles. C2h4 Molecular Geometry What is the shape of the molecule and the binding angle of C2H4. They say both shape and angle here.
Can I draw resonance structures of C 2 H 4 from the lewis structure of C 2 H 4. The ethylene is a covalent bond and lewis base. C2H4 Lewis Structure.
C2H4 has the Lewis Structure. C2H4 Lewis Structure Ethylene Welcome to Geometry of Molecules and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure of C2H4 molecule. A Draw the Lewis structures for C 2 H 4 and C 2 F 4 and give the ideal HCH and FCF bond angles.
There is a formation of a sigma bond and a pi bond between two carbon atoms. Ethane Hybridization Molecular Geometry and shape. Ethylene C 2 H 4 and tetrafluoroethylene C 2 F 4 are used to make the polymers polyethylene and polytetrafluoroethylene Teflon respectively.
It is a colorless and odorless molecule that exists as a gas at the standard room temperature. B The actual HCH and FCF bond angles are 1174 and 1124 respectively. Lewis structures polarities VSEPR geometries and bond angles These species will be explored.

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h6 Molecular Geometry Shape And Bond Angles Youtube
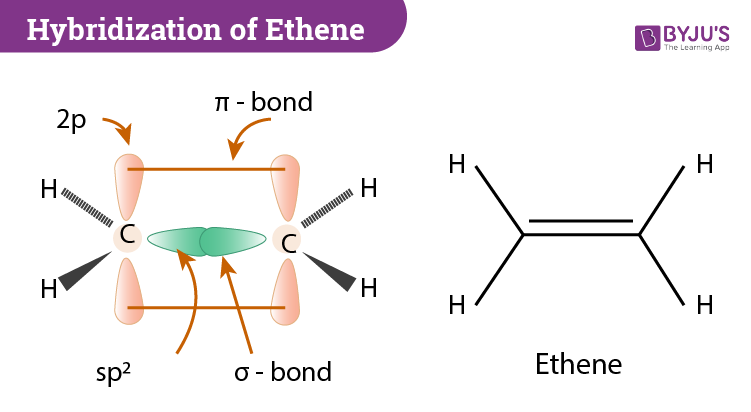
Hybridization Of C2h4 Ethene Hybridization Of Carbon In C2h4

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar
What Is The Hybridization And Bond Angle Of A C2h4 Molecule Quora
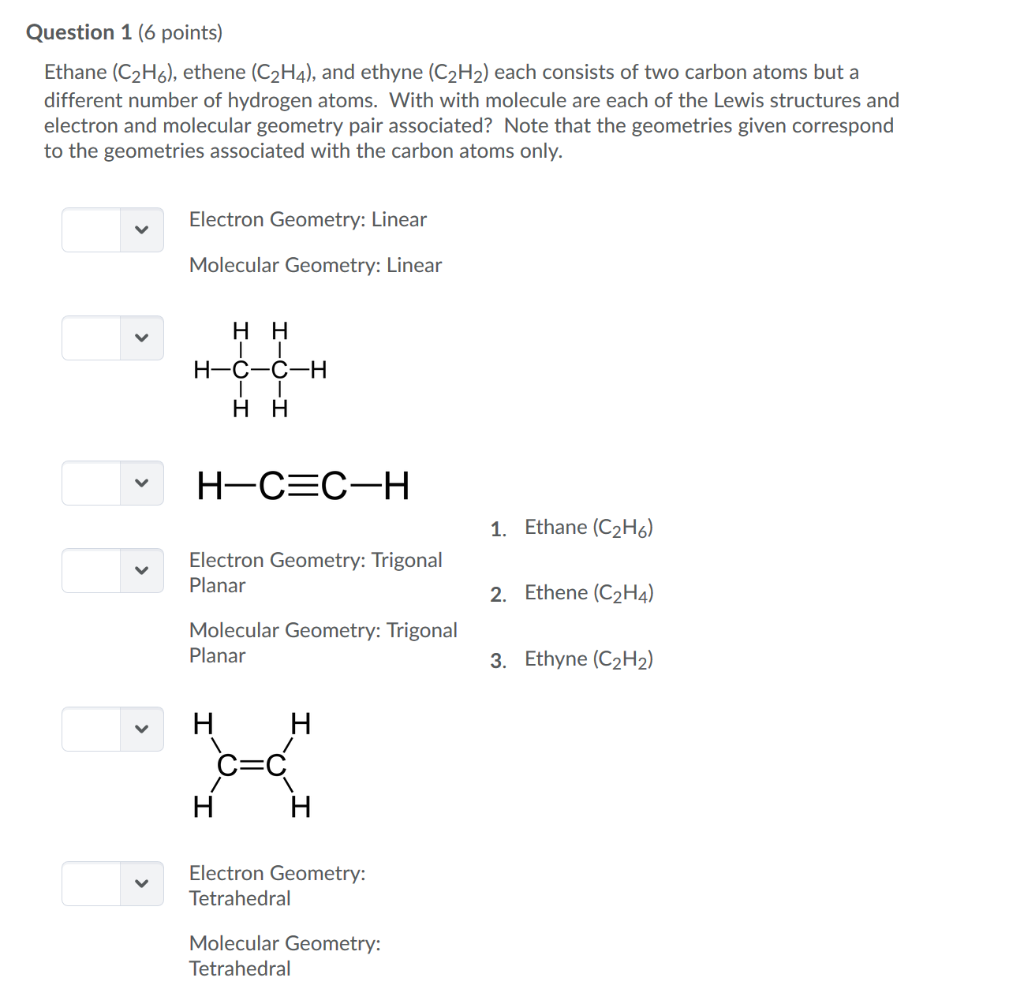
Question 1 6 Points Ethane C2h6 Ethene C2h4 Chegg Com
How Is C2h4 Planar While C2h6 Is Non Planar Quora
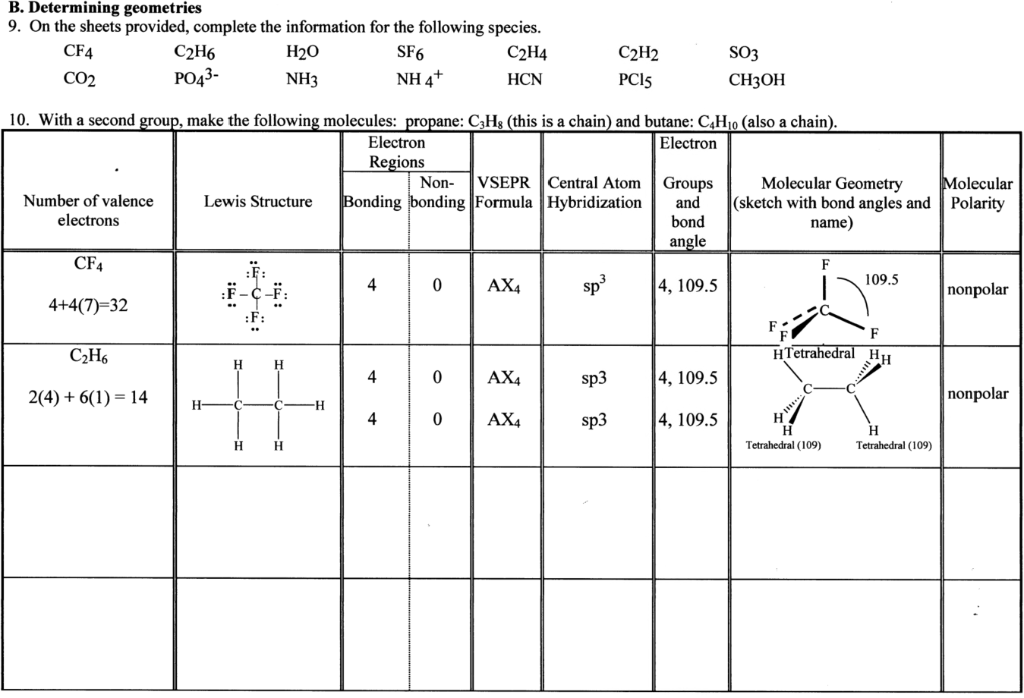
B Determining Geometries 9 On The Sheets Provided Chegg Com

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Observation 1 Geometries Of Molecules Chemistry Libretexts

Chemistry Molecular Structure 15 Of 45 Basic Shapes Predict The Shape Of C2h4 Youtube

C2h4 Molecular Geometry Shape And Bond Angles Youtube
C2h4 Molecular Geometry Shape And Bond Angles Youtube

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Wn C2h3f Lewis Structure Polar Or Nonpolar Bond Angle Molecular Geometry Hybridization
How To Draw Lewis Structure For C2h4 Drawing Easy
C2h4 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape
Molecular Geometry Predicted By Vsepr Ppt Download

Ch 10 Vsepr Practice Problems 3 Flashcards Quizlet