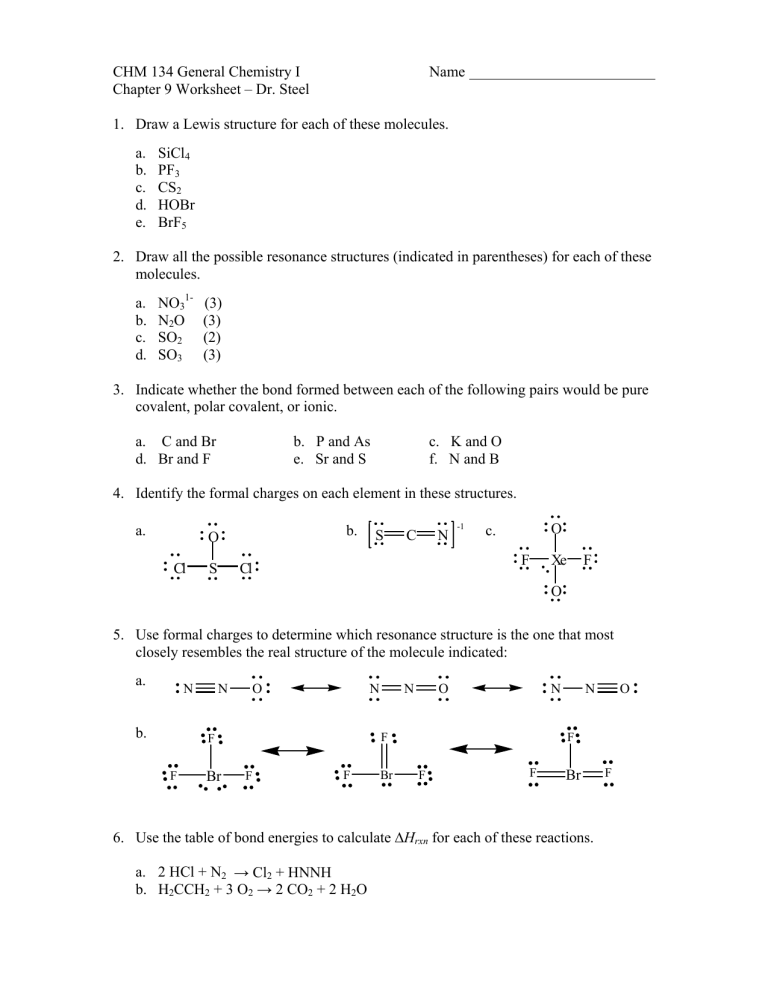
Brf5 Lewis Structure Formal Charge
Were just going to put those right here on the Bromine. You are asking for the Lewis Structure of a compound that cannot exist.

Brf5 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle
THIS SET IS OFTEN IN FOLDERS WITH.

Brf5 lewis structure formal charge. Chemistry questions and answers. The formal charge on Bromine atom of. What is the formal charge in BrF5 lewiss structure and how to calculate it.
Is XeF2 a Lewis structure. Uranium is converted to uranium hexafluoride by strong oxidizing agents including BrF5. Is BrF5 a dipole moment.
The formal charges in a structure tell us the quality of the dot structure. BF5 is Boron Pentafluoride and remember that Boron Pentafluoride has 3 electrons on its outermost shell P-shell. Each one of these 3 electrons has the potential to form.
HUMAN EXPOSURE AND TOXICITY. To determine the hybridization we take a look at the Lewis structure of the BrF 5 molecule. Formal Charge 7- 05 2 -6 0.
Let us calculate for BrF3. Bromine is in period 4 on the periodic table and it can have more than 8 valence electrons. Possible Formula Number of Lewis Structure Valence electrons Formal charge on central atom resonance structure Yes or No H2 PF3 N2 C2H4 CO2 AIC13 PO43- 13.
But on the other hand BrF5 does have a dipole moment due to the asymmetric structure as shown earlier in the figures. Formal charge Valence electrons unbonded electrons 12 bonded electrons. To calculate the formal charge on central bromine atom of BrF5 molecule by using the following formula.
The formal charge is a hypothetical charge assigned from the dot structure. The formal charge generally represents the actual charge on an individual atom of any molecule. In Lewis Structure formation we have to check whether all the atoms have their least possible formal charge values.
Some practice of assigning formal charge is necessary before you master this technique. To calculate the formal charge. There are a total of 22 valence electrons in the Lewis structure for XeF2.
To calculate the formal charge in CF4 lewis dot structure. The reaction of 20 g of Br2 with 40 g of F2 produced BrF5 in 45 yield. Use this equation given below.
Both Lewis structures have a net formal charge of zero but the structure on the right has a 1 charge on the more electronegative atom O. For the BrF5 Lewis structure the total number of valence electrons found on the periodic table is 42. Note that in the Lewis structure for BrF5 Bromine B is in Period Four on the periodic table.
Bromine Pentafluoride comprises 5 Fluorine atoms all pulled together by the central Bromine atom. Lewis Structure of PCl5 Lewis structure of a compound is the arrangement of its underlying atoms valence shell electrons. In the charge minimized Lewis structure of BrF4 the formal charge on any two fluorine atoms could be.
How many grams of BrF5 were obtained. The formal charge will be found on the central bromine atom of the BrF5 Lewis dot structure in the following calculation. Lewis structures make the use of dots to represent electrons and bonds between different electrons are represented through a straight line marked at the end of which is a set of electrons.
Molecule Valence e- Lewis Structure Molecular Shape Formal Charge Polar Molecule. This is how we calculate the formal charge. We will find the formal charge on the central atom of the BrF5 lewis dot structure which is bromine.
Thus the symmetrical Lewis structure on the left is predicted to be more stable and it is in fact the structure observed experimentally. Formal charge Nve. Metal chlorides bromides and iodides are converted to fluorides by treatment with BrF5.
Acute exposure to BrF5 can cause redness and tearing of the eyes. Formal Charge is the charge given to constituent atoms inside a chemical molecule where the bonding is shared equally among all the atoms present. If you were to check the formal charges for this structure youd see that the formal charge for each atom in BrF5 is zero.
NO3- SO42- BrF5 C2H2 XeCl4. BrF5 is predominantly used as a fluorinating agent to produce fluorocarbons and as an oxidizer in rocket propellant systems. Resonance SF4 XeF4 ClF3 BrF5 PF5 SF6 XeF2.
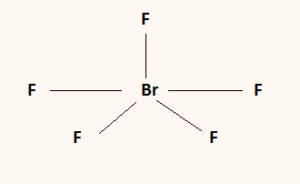
The formal charges being 0 for all of the atoms in the BrF 5 molecule tells us that the structure shown above is stable. So this is the Lewis structure for BrF5. BrF5 or bromine pentafluoride has a square pyramidal structure as in the first figure.
Once we know how many valence electrons there are in BrF5 we can distribute them around the central atom with the goal of filling the outer shells of each atom.
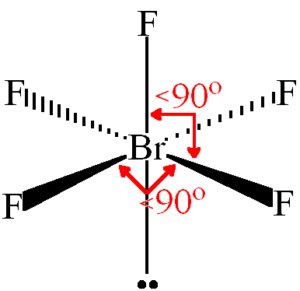
Brf5 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
Brf5 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape
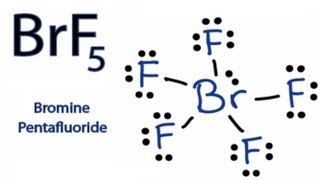
How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

General Chemistry Chemistry 1a Ch 10 The Shapes Of Molecules Ppt Download

The Central Atom In Brf5 Has How Many Bonding Pairs Of Electrons And How Many Non Bonding Pairs Of Electrons Study Com

Bromine Pentafluoride Brf5 Lewis Dot Structure Youtube

Lewis Structure Of If5 Or Brf5 Ibr5 Icl5 Brcl5 Youtube

Is Brf5 Polar Or Nonpolar Molecular Geometry Of Brf5

Brf5 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

Brf5 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

Name Section 1 Draw A Lewis Structure For Each Of Chegg Com
How To Determine The Lewis Structure For Pcl5 Quora
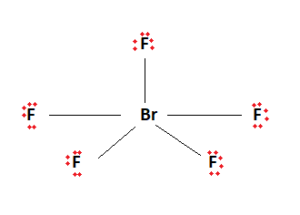
Brf5 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape
Brf5 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube
Lewis Structure Of If5 Or Brf5 Ibr5 Icl5 Brcl5 Youtube