How Many Lone Pairs In Xef4
These pairs adopt an octahedral arrangement. The shape of the I3 molecule is linear.
How Many Pairs Of Electrons Are There In The Lewis Structure Of Xef4 Quora
If the number is 4 there are two lone pairs on the central atom.
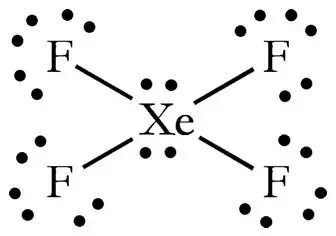
How many lone pairs in xef4. The XeF4 xenon tetrafluoride molecule is hypervalent with six electron pairs around the central xenon Xe atom. What are the number of lone pairs of electrons on SF4 CF4 and XeF4. Four of the pairs are bonding pairs and two are lone pairs.
36-8 28e-14 lone pairs Use information from step 4 and 5 to draw the lewis structure. We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more no less. Draw a correct Lewis structure for BF 3.
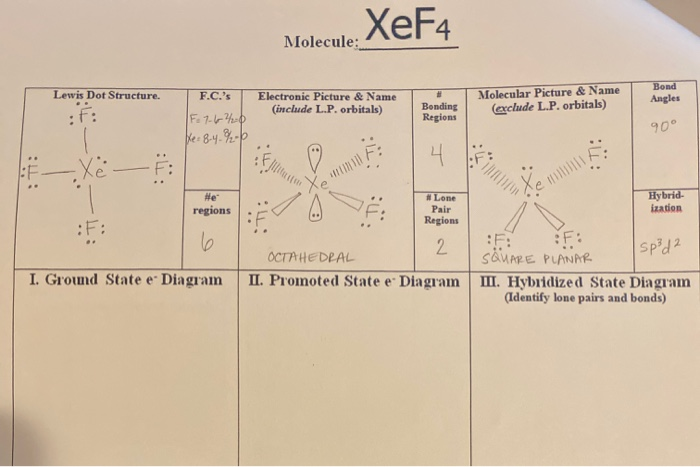
A step-by-step explanation of how to draw the XeF4 Lewis Dot Structure Xeon TetrafluorideFor the XeF4 structure use the periodic table to find the total n. The electron geometry is octahedral while the molecular geometry is square planar Xenon has 6 bonding electron pairs therefore the electron geometry of octahedral but two of the pairs of electrons on the central atom are unbonded or lone pairs therefore the molecular geometry is. So 3 2 5 also determines sp3d hybridization.
The central atom has no lone pair and there are four bond pairs. Lewis dot structure of XeF4 Alternatively a dot method can be used to draw the lewis structure. If the number is 0 there are no lone pairs on the central atom.
The number of lone pairs of electrons on Xe in X eF 2X eF 4 and X eF 6 are respectively are 3 2 and 1. What is the value of the bond angles in ccl4 enter the bond angle of the molecule. SF4 molecule has 1 lone pair of electrons CF4 has no lone pair of electrons and XeF4 has 2 lone pair of electrons respectively.
Hence there are a total of 36 valence electrons in XeF4. B in middle surrounded by Fs that puts a 0 formal charge on all atoms. Access a diverse Question Bank and ask You Own Doubt Now.
There are three iodine atoms one of which is also negatively charged. The Lewis structure of CCl4 is. The central atom in XeF4 is surrounded by a3 single bonds 1 double bond and no lone pairs of electrons.
Chlorine atoms establish covalent connections with the sulfur atom as a result leaving the sulfur atom with one lone pair. The Lewis structure for XeF4 has a total of 36 valence electrons. First try to figure out how many bond angles you need to determine for XeCl FXeF bond angles 90 or 180 Lone pairs are on opposite sides of the molecule 180 from each other to minimise lone-pairlone-pair interactions.
According to VSEPR theory the electronic repulsion of the lone pair and bond pair leads the SCl4 molecule to take on a. If the number is 2 there is one lone pair on the central atom. So there are 12 lone pairs on F atoms.
But in hybridization diagrams finding steric number detecting isostructural compounds etc the lone pairs on the main atom are taken ie. Draw a correct Lewis structure for XeF 4. So there are total 14 lone pairs on the molecule of XeF4.
Since there are 4 F atoms and each F atom has 3 lone pairs total lone pairs are 34 12. Hope my answer helps. Subtract step 3 number from step 1.
4 bonds and 0 lone pairs. There are already 4 sigma bonds in the above drawn basic sketch. The molecule has a grand total of 36 electrons.
Mark lone pairs on xenon and fluorine atoms After deciding the center atom and basic sketch of XeF4 we can start to mark Remember that there are total of 18 electron pairs to mark on atoms as lone pairs and bonds. How many lone pairs unshared pairs are on the central atom. Taking all in consideration 21214.
What is the value of the bond angles in XeF4. Click hereto get an answer to your question In XeF2 XeF4 and XeF6 the number of lone pairs of Xe is respectively. Here Xe so 2 lone pairs will be considered.
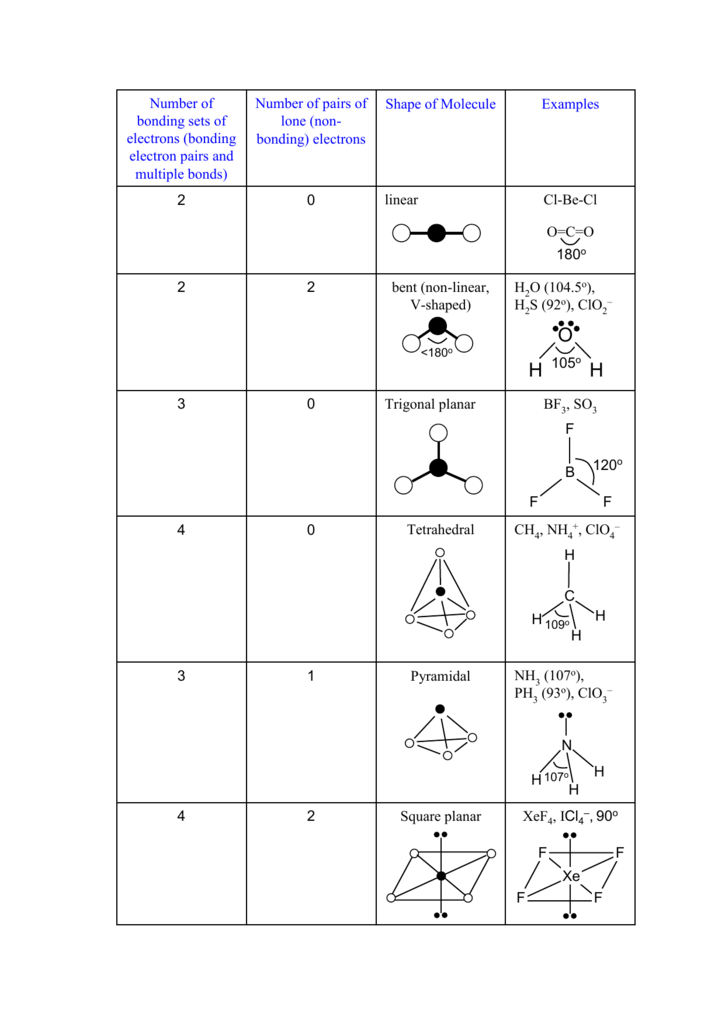
Xenon tetrafluoride XeF4 is a square planar non-polar molecule. The Xenon atom has 4 bonding pairs of electrons and 2 lone non-bonding pairs of electro. Xe in middle surrounded by Fs that puts a 0 formal charge on all atoms.
There is one lone pair on the sulfur central atom that resist the bond pairs of the four S-Cl. 3 lone pairs on each chlorine 2 lone pairs on the central iodine and 8 bonding electrons. Is XeF4 polar or non-polar.
When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. How many lone pairs are in SiCl4. Carbon is surrounded by 4 electron groups.
Is done on EduRev Study Group by JEE Students. The number of lone pairs of electrons in this molecule is 3 and the number of atoms with valence electrons is 2. The bond angles are 90 or 180.

Molecular Geometry Of Xef4 Youtube

The Total Number Of Lone Pair Present In Xef4 Brainly In

Number Of Lone Pairs And Bonding Pairs For Xef4 Xenon Tetrafluoride Youtube

21 Calculate The Number Of Lone Pairs On Central Atom In The Xef4 Molecule And Predict The Geometry On The Basis Of Vsepr Theory
Xef4 Molecule Lewis Dot Structure F C S Electronic Chegg Com

Xef4 Xenon Tetrafluoride Sp3d2 Hybridization Structure Shape Bond Angle Lone Pairs Adichemistry Youtube

Xef4 Lewis Structure And Molecular Geometry Youtube

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
What Is The Vsepr Structure Of Xef4 Quora
Xef4 Icl4 90o Square Planar 2 4 Nh 107o Ph 93o Clo

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 Youtube
Lewis Structure Of Xef4 Biochemhelp
How Many Numbers Of Lone Pair Is Present In Xef4 Quora
How Many Numbers Of Lone Pair Is Present In Xef4 Quora
What Is The Vsepr Structure Of Xef4 Quora

How To Find Total Number Of Lone Pair Present In Xef4 Brainly In

The Total Number Of Lone Pair Present In Xef4is 1 10 2 12 3 14 4 16 Brainly In

How Many Numbers Of Lone Pair Is Present In Xef4 Quora

How Many Numbers Of Lone Pair Is Present In Xef4 Quora