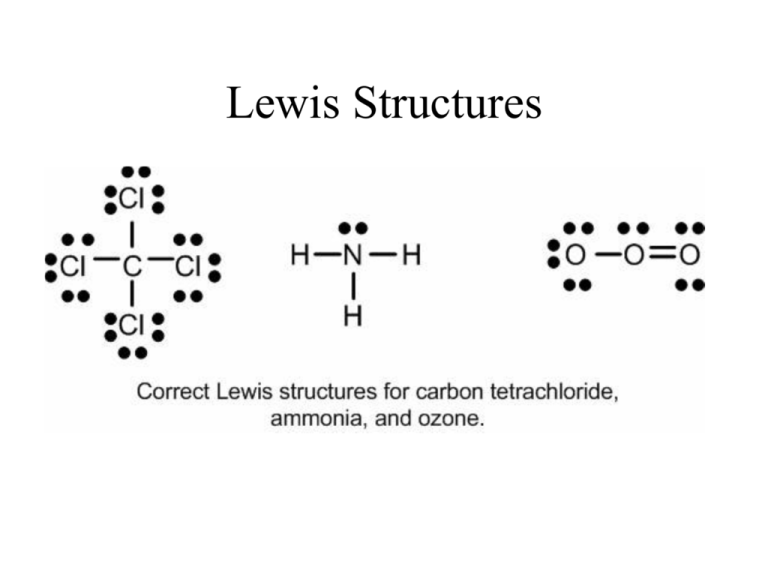
Lewis Dot Structure Of Co2 And Nh3
Analyze bond angles and bonding pairs. This means that all attraction forces inside the molecule rely on weak.

What Would Be The Electron Dot Structure Of Carbon Dioxide Which Has Formula Co Sub 2 Sub Chemistry Q A
Lets see how to draw lewis dot structure for NF3 with easy steps.

Lewis dot structure of co2 and nh3. Simple steps for drawing the Lewis dot structure for NF3. This increases electron-electron repulsion and therefore creates a bent structure as opposed to CO2s linear structure. It is a stable pnictogen hydride where all the atoms are covalently bonded to achieve a reactive state.
A step-by-step explanation of how to draw the NH3 Lewis Dot Structure AmmoniaFor the NH3 structure use the periodic table to find the total number of vale. This structure is very similar to NCl3 and NH3. Generally small symmetric molecules are nonpolar.
Which of these molecules is polar. Ammonia NH 3 is a colorless pungent gas and is made up of nitrogen and hydrogen. Lewis structure electron dot diagram for ammonia or note that there are 3 covalent bonds 3 bonding pairs of electrons in total and that there is a lone pair non bonding pair of electrons on the nitrogen atom.
The carbon is sp hybridized and oxygens are sp2 hybridized. HCN NH3 O CO2 1 poil QUESTION 2 TrueFalse. There is a total of 10 lone pairs and 3 bonded pairs present in the NF3 lewis structure.
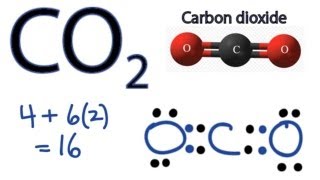
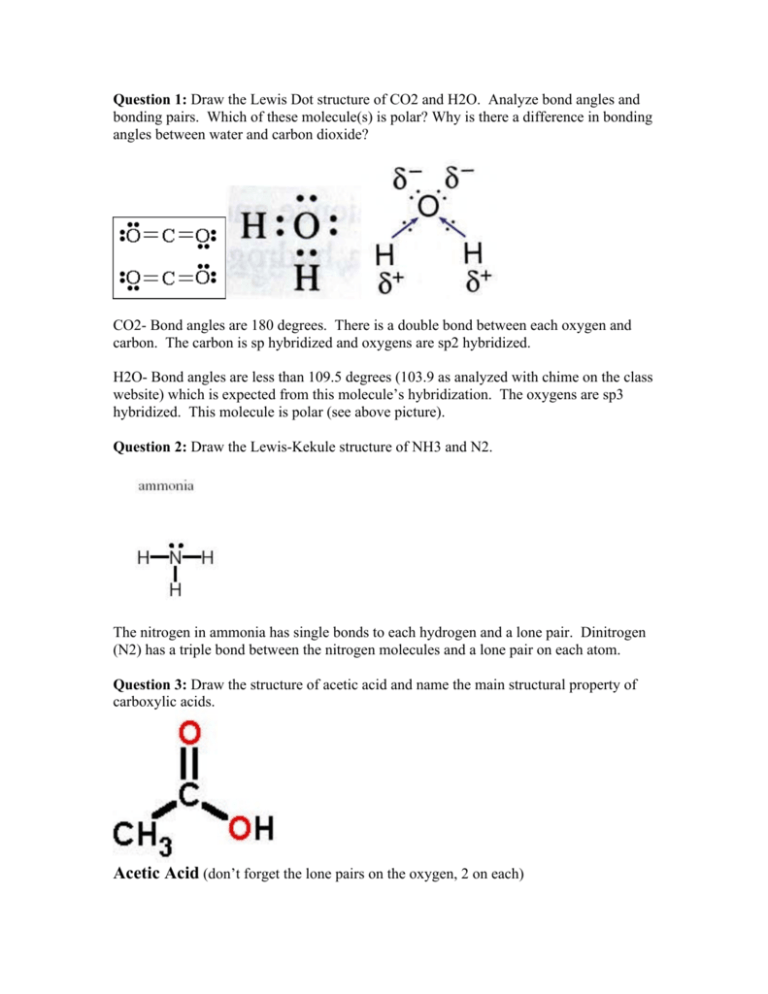
All tetrahedral molecules are non-polar. NH3 molecular geometry is of pyramidal shape with triangular pyramidal geometry. There is a double bond between each oxygen and carbon.
H2Os Lewis Dot Structure gives it many unique properties mostly due to the two lone pairs on the central oxygen atom. Which of them are polar. 1S 2 2S 2 2P 3.
In Lewis dot structures each dot. CO 2 has a rather low boiling point of around -80 or -100. Ammonia is the simplest binary hydride made up of nitrogen and hydrogen denoted by its chemical formulae as NH3.
NH3 Lewis Structure In Lewis structureit is common that a bonding pair of two electrons can be shown by dash- or dots but a lone pair of two electrons is shown by dots. In the NH3 Lewis structure three hydrogen atoms are bound to a nitrogen atom. Net Add Momentum Digital Animation 1000 15 Verizon.
Amy Schumer talks period myths and Tampax tampons. Lewis Dot Structure of CO2 Carbon DiOxide - YouTube. Why is there a difference in bonding angles between water and carbon dioxide.
But each oxygen in the CO2 lewis dot structure has two lone pairs. One of the most fascinating phenomena is the idea of hydrogen. Consider CO2 NH3 and HCN.
Ammonia is lighter than the air colorless and pungent in smell. Draw the Lewis Dot structure of CO2 and H2O. It is a pictorial representation of the arrangement of valence electrons around the individual atoms in the molecule.
Ammonia nh 3 is a commonly tested lewis structure due to its widespread use in agriculture as a fertilizer. Its used in the chem lab when. NH3 Lewis structure ammonia electron dot structure is that type of diagram where we show the total eight valence electrons of NH3 as dots or dots and dashes-.
Count total valence electron in NF3. NH3 Lewis Structure Geometry and Hybridization. The Lewis dot structures are drawn for you on page 12 of your Chapter 4 Pt.
It can be liquified and even frozen solid with special machinery to produce dry ice Dry ice exhibits a temperature around the boiling point mentioned previously. This Lewis Dot Structure also explains some of the fundamental properties of this particle. A lewis diagram helps us to know how electrons are arranged around individual atoms in a molecule.
NH4OH Lewis Dot Structure Ammonium Hydroxide - YouTube. Lewis defined a base as an electron pair donor and an acid as an electron pair acceptor. This bent molecular structure gives it many unique properties such as being polar.
The CO 2 Lewis structure is symmetric. NF3 lewis structure has 3 fluorine and 1 nitrogen atom connected with three single bonds. CO2 lewis dot structure contains two oxygen atoms and one carbon atom connected with the double bond whereas carbon is the central atom and no lone pair is present on it.
Choose anyall that apply. Since there are no lone pairs on the central atom or any atom for that matter there are few dipoles created and the minimal electronegativity difference means that these bonds can essentially be treated as nonpolar covalent bonds. Lewis dot structures reflect the electronic structures of the elements including how the electrons are paired.
CO2- Bond angles are 180 degrees. Ammonia or NH3 has a total of 8 valence electrons. CO 2 is a nonpolar substance meaning it tends to be a gas.
Lewis structures are a useful way to summarize certain information about bonding and may be thought of as electron bookkeeping. The Lewis structure of a molecule helps understand the electron geometry molecular geometry polarity and other such properties with ease. CH4 Lewis Structure - How to Draw the Dot Structure for CH4 Methane - YouTube.
Lewis dot structures are commonly referred to as electron dot structures or Lewis structures. True False 1 poin QUESTION 3 Consider the hypothetical molecules YX2 and AZ2 whose Lewis dot structures.
Co2 Lewis Structure Molecular Geometry And Hybridization

Co2 Lewis Structure Carbon Dioxide Youtube
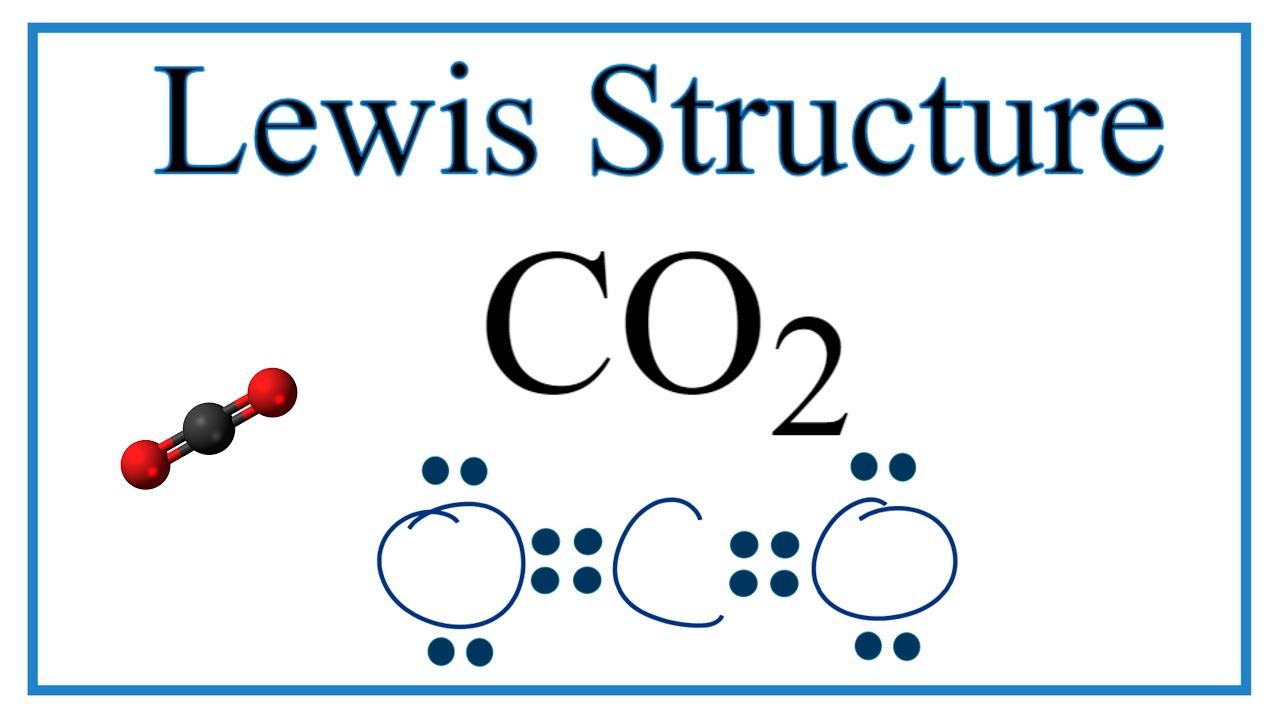
Type Of Bonds For Co2 Carbon Dioxide Youtube

Carbon Dioxide Lewis Structure How To Draw The Lewis Structure For Carbon Dioxide Youtube

Show The Formation Of The Following Molecules With The Help Of Electron Dot Structure A Ch4 B Co2 Brainly In

Write The Electron Dot Structure Of Co2 H2o H2s Propane F2 Ch4 Brainly In
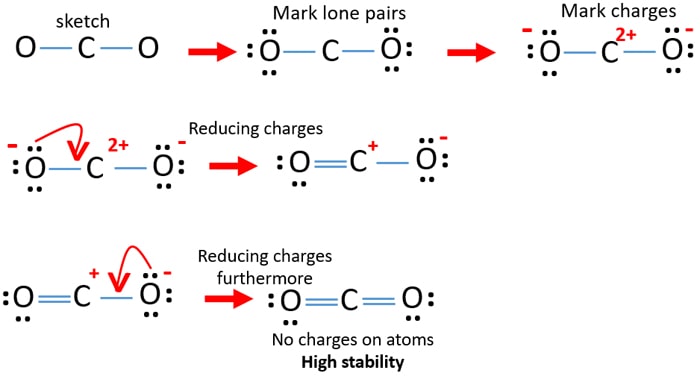
Co2 Carbon Dioxide Lewis Structure And Shape
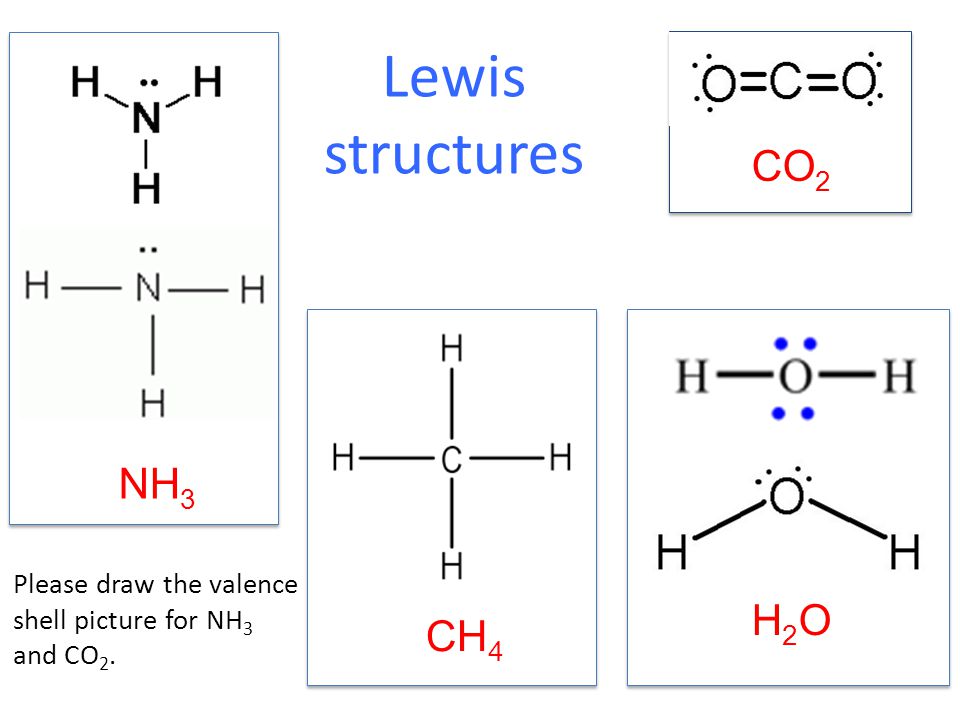
Lewis Structures And Vsepr Ppt Video Online Download

Write The Electron Dot Structure Of Co2 H2o H2s Propane F2 Ch4 Brainly In

Draw Electron Dot Structure Of Co2 And Nh3 Brainly In
Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube

Nh4 Lewis Structure Ammonium Ion Youtube

Draw The Electron Dot Structure Of Carbon Dioxide Co2 Brainly In

Draw Electron Dot Structure Of Co2 And Nh3 Brainly In

Draw The Electron Dot Structure Of Co2 Ch4 S8 Brainly In
Question 1 Draw The Lewis Dot Structure Of Co2 And H2o Analyze

Draw The Lewis Structures For The Following Molecules And Ions H2s Sicl4 Bef2 Co 2 3 And Hcooh

Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube