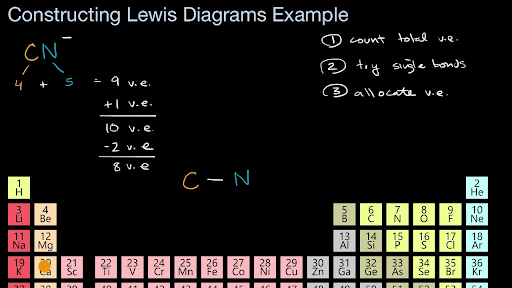How Many Lone Pairs Are In The Best Lewis Structure Of Cn-
The formula for the cyanide ion is C N X. As per CF4 lewis structure no lone pair present on the central atom carbon.
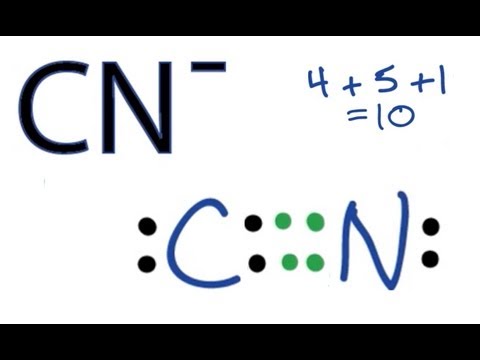
Cn Lewis Structure How To Discuss
This liquid is used in electroplating mining and as a precursor for several compounds.
How many lone pairs are in the best lewis structure of cn-. Draw Lewis structure for CN CN and CN- and include formal charges lone pairs and nonbonding electrons. Each S has 1 S - Cl single bond. This problem has been solved.
Like that three oxygen atom will take six 6 lone pairs. HCN Lewis Structure Molecular Geometry Shape and Polarity. See the answer See the answer See the answer done loading.
As the next step mark those six valence electrons pairs on outside atoms nitrogen and sulfur atoms as lone pairs. A step-by-step explanation of how to draw the CN- Lewis Dot Structure Cyanide ionNote. There are only two 2 bonds around center atom in the sketch of thiocyanate ion.
A step-by-step explanation of how to draw the NO Lewis Dot Structure Nitrosonium ionFor the NO structure use the periodic table to find the total number. This chemistry video tutorial provides a basic introduction into drawing lewis dot structures but most importantly it provides an explanation on how to calc. There are two obvious ways to build the Lewis structure.
Draw a Lewis structure for SO2 in which all atoms have a formal charge of zero. Tries 03 How many lone pairs are in the best Lewis structure of SeCN. So carbon is the central atom that attached to four fluorine atoms with the help of a single bond and it has 4 valence electrons in its outermost shell.
Or you can check the lone pair on the central atom by the formula given below-. The SCN- Lewis structure is a good structure to help you understand. In that second example the nitrogen has two lone pairs.
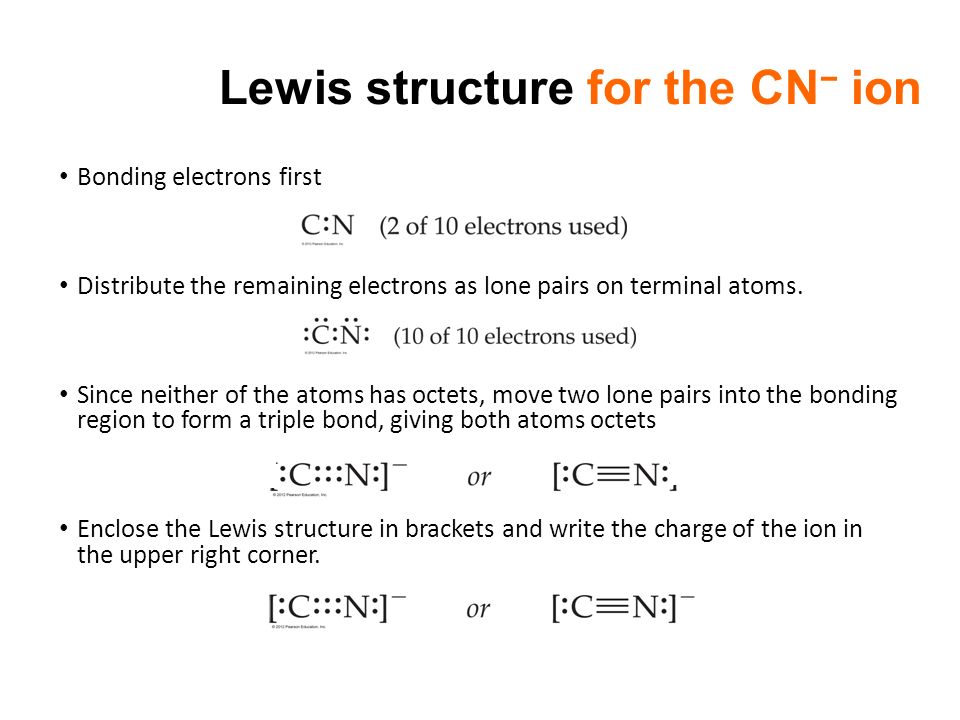
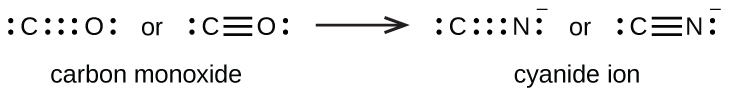
1 S - S single bond. The 8 electrons total 5 from nitrogen 1 from each of the 4 hydrogens minus 1 which creates the positive charge perfectly fill all of their outer orbitals with no lone pairs. CN- however contains a triple bond between carbon and nitrogen with lone pairs on both atoms totaling to 10 electrons 4 from carbon plus 5 from nitrogen plus the extra electron which creates the negative charge.
Now three 9-6 lone pairs are remaining and they are marked on other oxygen atom. I hope that was clear. Formal Charge Practice Problems Q.
Im not sure how to make ASCII Lewis structures. Now all lone pairs are finished and no lone pairs to mark on phosphorous atom. Each oxygen atom which have joint with a hydrogen atom will take two lone pairs.
Therefore now six 8-2 electrons pairs are remaining to mark lone pairs. How many lone pairs are in the best Lewis structure of CO. There should be brackets around the final Lewis Structure with a -1.
Hydrogen Cyanide is a colorless flammable and poisonous chemical liquid. A correct Lewis structure for C2H2Cl2 would have what bonds. All Chemistry Practice Problems Lewis Dot Structure.
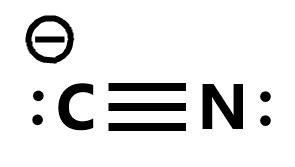
There is two lone pair present one on nitrogen and the other on carbon. In this video were going to try to get more practice constructing Lewis diagrams and were gonna try to do that for a cyanide anion so this is interesting this is the first time were constructing a Lewis diagram for an ion so pause this video and see if you can have a go at that alright now lets do this together so weve already seen in many videos the first step is to essentially count the. What is the total number of valence electrons in the Lewis structure of CN-.
Carbon C-O and C-S. Represented by the chemical formula HCN is one of those molecules that has an interesting Lewis structure. A step-by-step explanation of how to draw the SCN- Lewis Structure Thiocyanate Ion.
A double bond between the 2 Cs with either 2 Cl on one C and 2 H on the other C or a Cl and an H on each C. That gives us a total of ten valence electrons to work with. What is the formal charge on the N atom in the correct or best Lewis structure for CN.
C N X. HD Tries 05 What is the total number of valence electrons in the Lewis structure of SeCN. Lone pairs on atoms.
Lewiss structure of cyanide CN- contains two atoms carbon and nitrogen connected with a triple bond. X C N.

Hcn Lewis Structure How To Draw The Lewis Structure For Hcn Youtube
Chapter 8 Basic Concepts Of Chemical Bonding Ppt Download
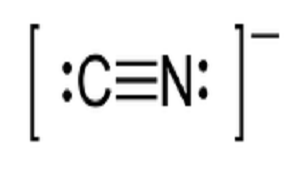
Solutions 17 Chemistry Libretexts

Draw Lewis Structure For Cn Cn And Cn And Include
10 1 Lewis Structures And The Octet Rule Chemistry Libretexts

Lewis Diagram Of The Cyanide Ion Worked Example Video Khan Academy

What Is The Correct Lewis Structure Of Diazomethane Chemistry Stack Exchange

Writing Lewis Structures Ppt Video Online Download

Draw A Lewis Structure For The Cyanide Ion Including Lone Pairs And Formal Charges Study Com

Cn Lewis Structure Cyanide Youtube

Makethebrainhappy The Lewis Dot Structure For Hcn
Lewis Diagrams Formal Charges How Many Lone Pairs Chegg Com
Cn Cyanide Ion Lewis Structure Molecular Geometry And Polarity Geometry Of Molecules
Lewis Diagram Of The Cyanide Ion Worked Example Video Khan Academy

How To Calculate The Formal Charges For Cn Cynide Ion Youtube
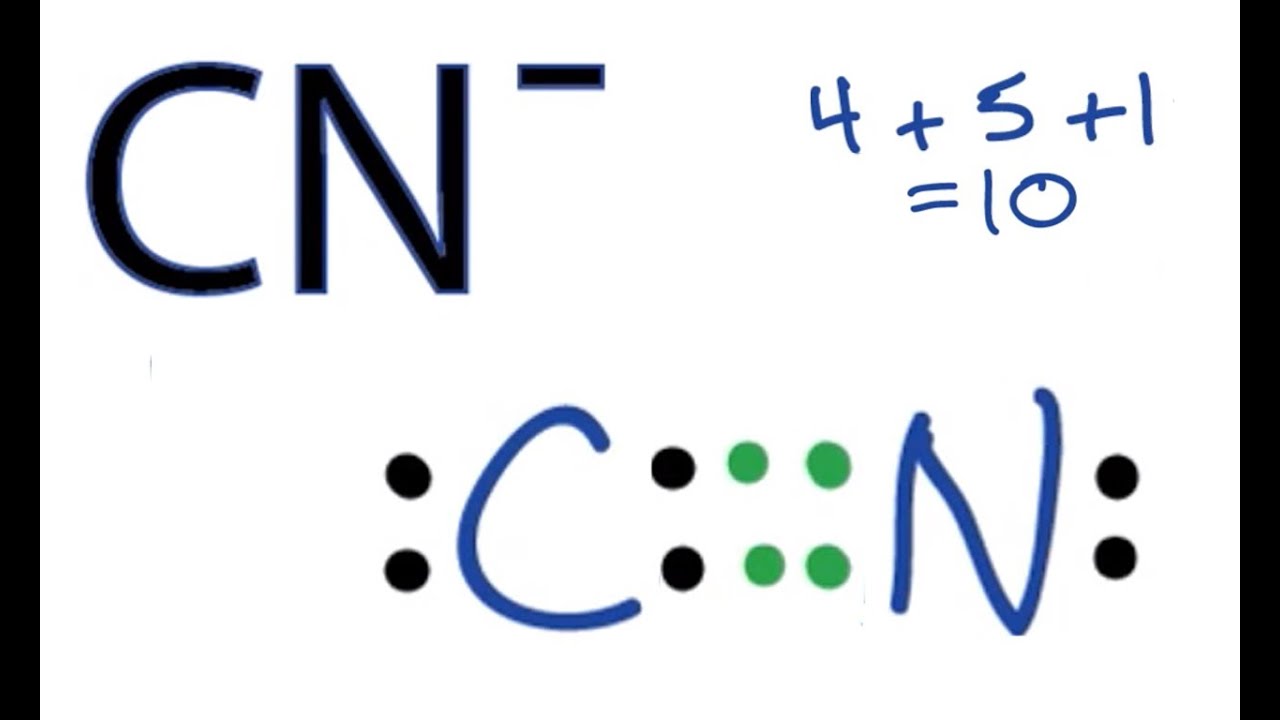
Cn Lewis Structure How To Draw The Dot Structure For Cn Chemical Bonding Success In Chemistry
Cn Cyanide Ion Lewis Structure Molecular Geometry And Polarity Geometry Of Molecules

Ocn Lewis Structure How To Draw The Lewis Structure For Ocn Youtube
What Is The Formal Charge On The N Atom In The Chegg Com