How To Find Hybridization Of Xef4
What would be the molar mass of a gas for which 243 x 10-2g occupies a volume of 768 mL at STP. Out of which two are in p-orbitals and the other two unpaired electrons are in d-orbitals.

Xef4 Lewis Structure And Molecular Geometry Youtube
By R Eisman 1990 Cited by 144 Platelet factor 4 PF4 is a 70 amino acid heparin-binding protein.

How to find hybridization of xef4. Sp hybridization is also called diagonal hybridization. To determine hybridization following rules. Therefore we say that Xe has a d 2 sp 3 hybridization.
In summary What is the hybridization of Xe in XeF4. These hybridized orbitals lead to sp3d2 hybridization in XeF4. How many of the following molecules have sp2d2 hybridization on the central atom.
Now if we count the number of valence shell in Xe we will find two electrons in. The Secret Science of Solving Crossword Puzzles Racist Phrases to Remove From Your Mental Lexicon. XeF4_hybridization XeF4_lewis_structure sp3d2_hybridizationThe structureshapemolecular geometry of Xenon tetrafluoride - XeF4 sp3d2 hybridization of XeF.
Starting with the of valence electrons 8 4 7 36 e - ie 18 pairs and using the VSEPR model we predict an octahedral arrangement of electron pairs about Xe. Is done on EduRev Study Group by JEE Students. As we know in the case of XeF₄ or xenon tetrafluoride the hybridization of xeof₄ occurs in the central atom which is Xenon Xe.
First try to figure out how many bond angles you need to determine for XeCl FXeF bond angles 90 or 180 Lone pairs are on opposite sides of the molecule 180 from each other to minimise lone-pairlone-pair interactions. Thereafter divide it by 8 dont write results in decimal form write it in quotient remainder form. The molecule XeF4 is a nonpolar molecule.
Of F atom is 4 so total valence electron becomes 7428. Valence electron of Xe 8 that of F7 here no. Write the Lewis structure.
Count valence electron veof each atom present in the compound but take valence electron of hydrogen as 7 Add them. By ID Page Prediction of sp sp2 sp3 Hybridization state Power on the Hybridization state of the central atom Total no of σ bonds around each central. Now we have to determine hybridization of XeF4.
For PF4 to chromosome 4q 1221 by. Hybridization of xef4. Seclo XeF4 IFs AsCls O 1 O2 3 4 OO In order to form an octet an atom of Rb will.
It is better to write the Lewis structural formula to get a rough idea about the structure of molecule and bonding. O gain 2 electrons O gain 6 electrons o lose 6 electrons o lose 2 electrons. The central Xenon atoms orbitals are hybridized which results in the formation of new hybridized orbitals.
In this case if we gaze upon the valence shell of Xe the total amount of electrons is six in the 5p orbital as well as two electrons in the 5s orbital. How to Calculate Hybridization Hybridization Of Sf6 Xef4 NH4 I3- PCl5 Bf3Welcome to my Channel Ajay Academy Friends es Channel pr aapko Easy ta. Quotient will represent bond.
Now X quotient remainder2426. It is a powerful fluorinating as well as an oxidizing agent. What is the hybridization of the central atom GN Lewis first proposed this theory in 1916 that helps in understanding the involvement of electrons informing the structure of the chemical.
Since there are 4 bonds and 0 lone pairs the hybridization will be sp3. BeCl2 BF3 SnCl2 CH4 NH3 H2O PCl5 SF4 BrF3 XeF2 SF6 IF5 XeF4 By. To find out the hybridization first you need to find steric number of the central atom by the formula steric no 12no.
Each sp hybridized orbital has an equal amount of s and p character ie 50 s. Calculate the number of lone. Calculate the number of sigma σ bonds.
This type of hybridization involves the mixing of one s orbital and one p orbital of equal energy to give a new hybrid orbital known as a sp hybridized orbital. Now 368 quotient 4remainder 4 Remainder 2422. A sigma bond is created in the process.
To find out the hybridization first you need to find steric number of the central atom by the formula steric no 12no. Adding we get 82836. As the geometrical structure of XeF4 is symmetric ie.
The remaining 4 unpaired electrons form the sp3d2 hybridization which consists of 2 unpaired electrons in the 5p orbital and 2 others in the 5d orbital. 5 EASY STEPS TO GET THE TYPE OF HYBRIDIZATION SHAPE. Xenon has six electrons in its 5p orbitals and.
Find the hybridization of central atoms for the following. Now looking on the table described above hybridization of XeF4 sp3d2. As a result there are four unpaired electrons in total.
Xe is in group 8 the noble gases. But when this atom is in an excited state two electrons in the p-orbitals move to d-orbitals. You would start by drawing the Lewis Dot Structure and then count the number of bonds and lone pairs on the central atom.
Given that volume of gas at STP V 768 mL 000768.

What Is The Hybridization Of Xef4 Quora

Brf3 Lewis Structure Bromine Trifluoride In 2021 Lewis Chemical Formula Dots

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Xef4 Molecular Geometry Bond Angles Electron Geometry Xenon Tetrafluoride In 2021 Molecular Geometry Molecular Geometry

Is Of2 Polar Or Nonpolar Oxygen Difluoride In 2021 Oxygen Chemical Formula Molecules

What Is The Hybridization Of Xef4 Quora
What Is The Hybridization Of Xef4 Quora
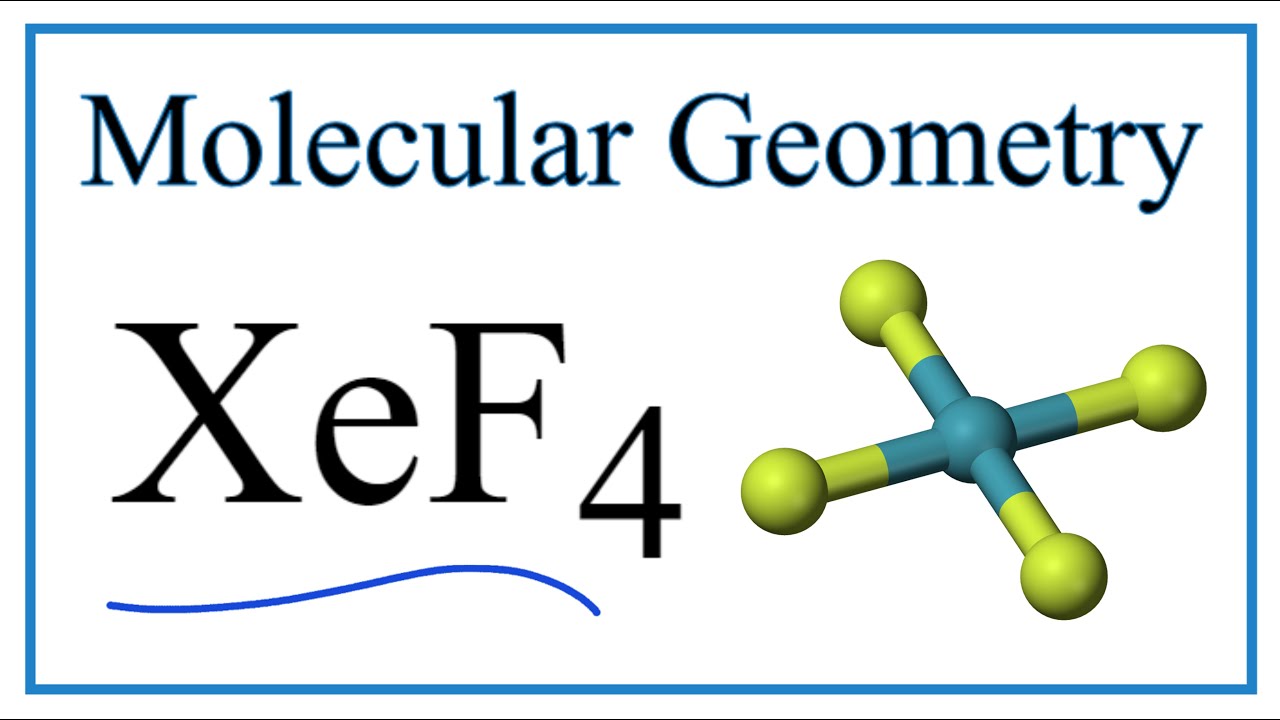
Xef4 Molecular Geometry Bond Angles Electron Geometry Youtube

Molar Mass Of Nh4cl Ammonium Chloride In 2021 Molar Mass Molars Molecules
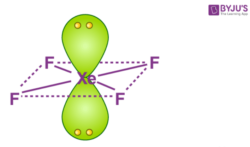
Hybridization Of Xef4 Hybridization Of Xe In Xenon Tetrafluoride

Is Ch3oh Polar Or Nonpolar Methanol In 2021 Functional Group Molecules Chemical Formula

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 Youtube
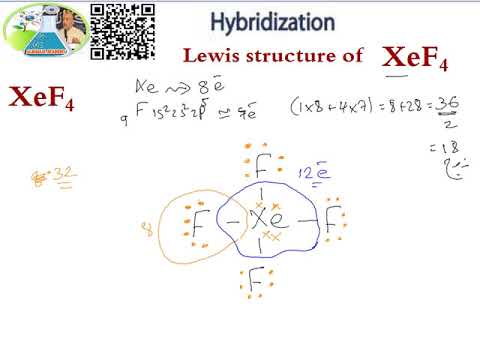
Lewis Structure Hybridization Xef4 Youtube

Hybridization Of Xef4 Xenon Tetrafluorid Youtube

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar

Q 31 Hybridization And Structure Of Xef4 Is Neet Practice Test 2 Vishal Academy Youtube

Xef4 Molecular Geometry Xenon Tetrafluoride Is A Chemical Compound With Chemical Formula Xef4 It Is Produced By Molecular Geometry Chemical Formula Geometry

Hybridization Of Xef4 Xenon Tetrafluorid Youtube

Xef4 Xenon Tetrafluoride Sp3d2 Hybridization Structure Shape Bond Angle Lone Pairs Adichemistry Youtube