The Correct Lewis Structure Of N2h2 Shows
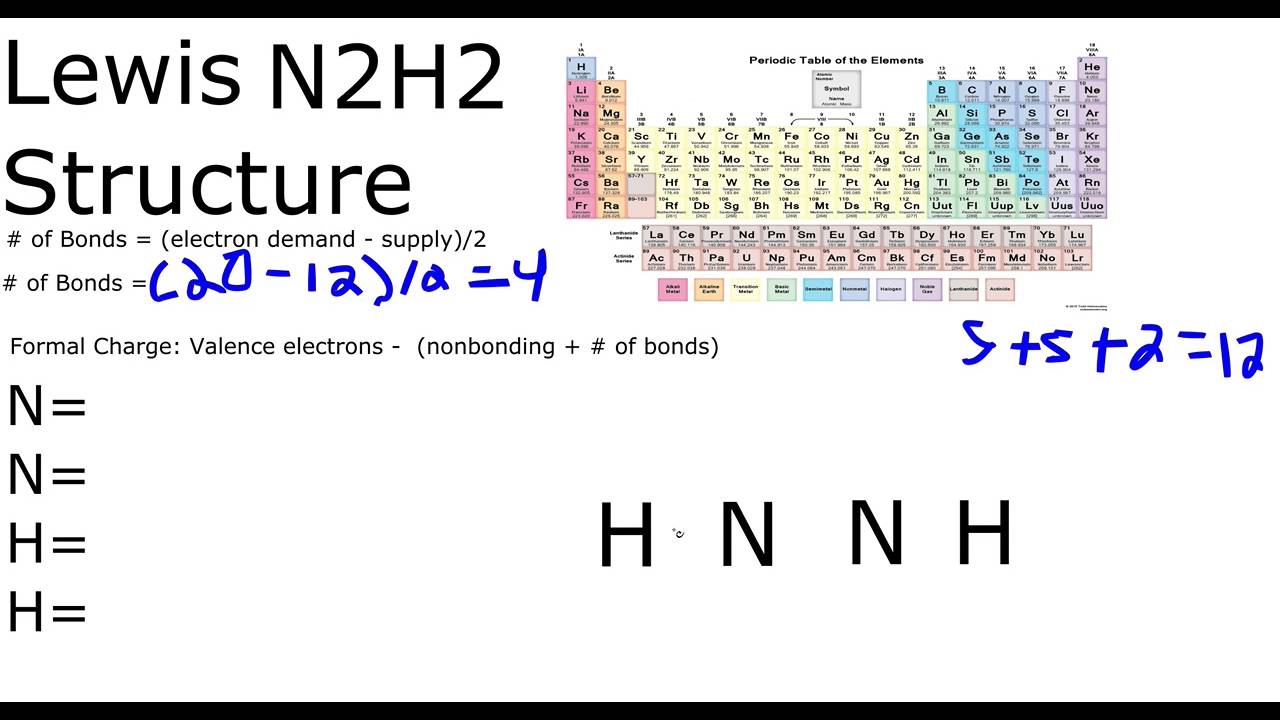
Total of 12 electrons. N has 5 5 10 electrons.
Question 2 The Lewis Structure Of N2h2 Shows Each Chegg Com
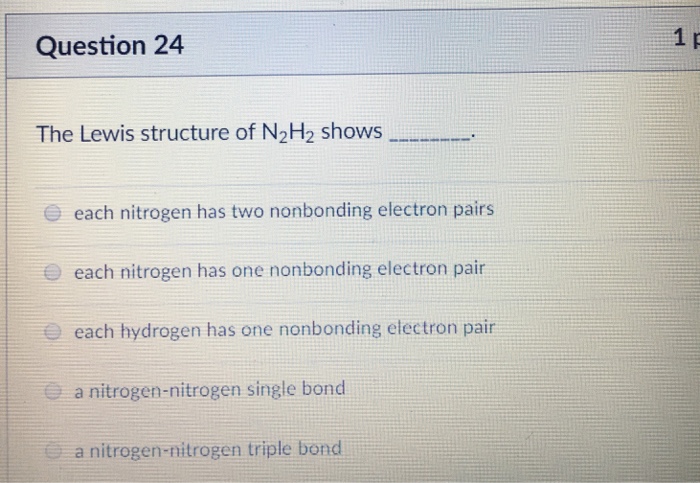
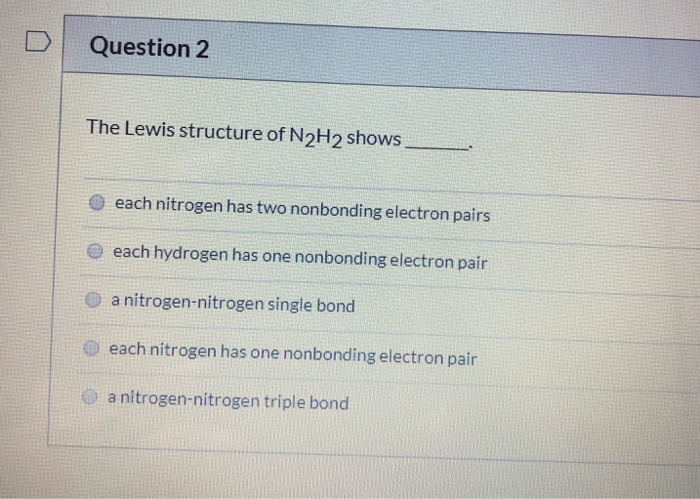
The Lewis structure of N2H2 shows _____.

The correct lewis structure of n2h2 shows. Four single bonds no double bonds and two lone pairs d. HNNH which accounts for 8. Write the Lewis structure for the diatomic molecule P 2 an unstable form of phosphorus found in high-temperature phosphorus vapor.
Two single bonds one double bond and two unshared pairs 2. That will add 4. A a nitrogen-nitrogen triple bond.
Let us help you simplify your studying. The Lewis structure of N2H2 shows _____. Include all lone pairs of electrons.
D each nitrogen has two nonbonding electron pairs. For example consider the ammonium ion NH 4 which contains 9 5 from N and 1 from each of the four H atoms 1 8. In the n 2 h 2 lewis structure the two nitrogen n atoms go in the center hydrogen always goes on the outside.
All of the above QUESTION 45 Draw the Lewis structure for TeF 4 that minimizes formal charges and select the correct description of the electrons around the central atom. View The Lewis structure of N2H2docx from CHEM 1331 at University of Houston. The Lewis structure of N2H2 shows _____A a nitrogen-nitrogen triple bondB a nitrogen-nitrogen single bondC each nitrogen has one nonbinding electron pairD each nitrogen has two nonbinding electron pairsE each hydrogen has one nonbonding electron pair.
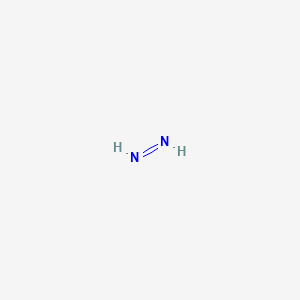
The Lewis structure for N 2 H 2 HHNH shows 1. Note that the H2O2 Lewis structure is frequently used on tests a. When the Lewis structure of an ion is written the entire structure is placed in brackets and the charge is written as a superscript on the upper right outside of the brackets.
Three single bonds and four unshared pairs 3. Two single bonds two double bonds and no lone pairs b. 13 The Lewis structure of N2H2 shows _____.
Group of answer choices a nitrogen-nitrogen single bond each hydrogen has one nonbonding electron pair each nitrogen has one nonbonding electron pair each nitrogen has two nonbonding electron pairs a nitrogen-nitrogen triple bond. A nitrogen-nitrogen triple bond a nitrogen-nitrogen single bond each nitrogen has one nonbonding electron pair each nitrogen has two nonbonding electron pairs each hydrogen has one nonbonding electron pair. I would distribute the 12 as follows.
The Lewis structure of N2H2 shows _. The Lewis structure of N2H2 shows _____. See full answer below.
Three single bonds and two unshared pairs. Each nitrogen has one nonbonding electron pair a nitrogen-nitrogen triple bond a nitrogen-nitrogen single bond each nitrogen has two nonbonding electron pairs each hydrogen has one nonbonding electron pair. In the Lewis structures listed below M and X represent various elements in the third period of the periodic table.
A a nitrogen-nitrogen triple bond B a nitrogen-nitrogen single bond C each nitrogen has one nonbonding electron pair D each nitrogen has two nonbonding electron pairs E each hydrogen has one nonbonding electron pair. The Lewis structure of N2H2 shows ________. The Lewis structure of N2H2 shows single bonds between the nitrogen and hydrogen atoms in the compound while the N atoms are connected via a double.
Three single bonds one double bond and two lone pairs C. The lewis structure for n2h2 hhnh shows1. E each hydrogen has one nonbonding electron pair.
C each nitrogen has one nonbonding electron pair. N2h2 lewis structure molecular geometry hybridization and mo diagram dinitrogen dihydride has the chemical formula of n2h2. Place a pair of unshared electron on the first N and a pair of unshared electrons on the second N.
A each hydrogen has one nonbonding electron pair B a nitrogen-nitrogen triple bond C each nitrogen has one nonbinding electron pair D each nitrogen has two nonbinding electron pairs E a nitrogen-nitrogen single bond. Our videos will help you understand concepts solve your homework and do great on your exams. A step-by-step explanation of how to draw the H2O2 Lewis Dot Structure Hydrogen peroxide.
Sep 7 2009. If you are having trouble with Chemistry Organic Physics Calculus or Statistics we got your back. Write the formula of each compound using the chemical symbols of each element.
B a nitrogen-nitrogen single bond. H has 1 1 2 electrons. Our videos prepare you to succeed in your college classes.
The Lewis structure of N2H2 shows _____.
Final 8 The Lewis Structure Of N2h2 Shows A Each Chegg Com

Draw The Molecules Include All Lone Pairs Of Electrons N2h2 N2h4 C2h2 C2h4 H3coch3 Study Com

The Lewis Structure Of N2h2 Shows A A Chegg Com
Question 24 The Lewis Structure Of N2h2 Shows Each Chegg Com

The Lewis Structure Of N2h2 Shows A Each Nitrogen Chegg Com

Welcome To Quilava S Blog Chemistry Education Structural Formula Nurse Drawing
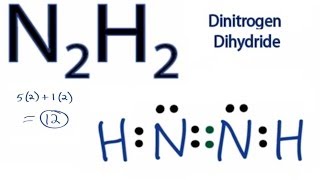
N2h2 Lewis Structure How To Draw The Dot Structure For N2h4 Chemical Bonding

Compare The N N Bond In The Molecules N2 And N2h2 Study Com

The Molecule Called Diazene Has The Formul Clutch Prep

The Lewis Structure Of N2h2 Shows A A Nitrogen Nitrogen Triple Bond B A Nitrogen Nitrogen Brainly Com
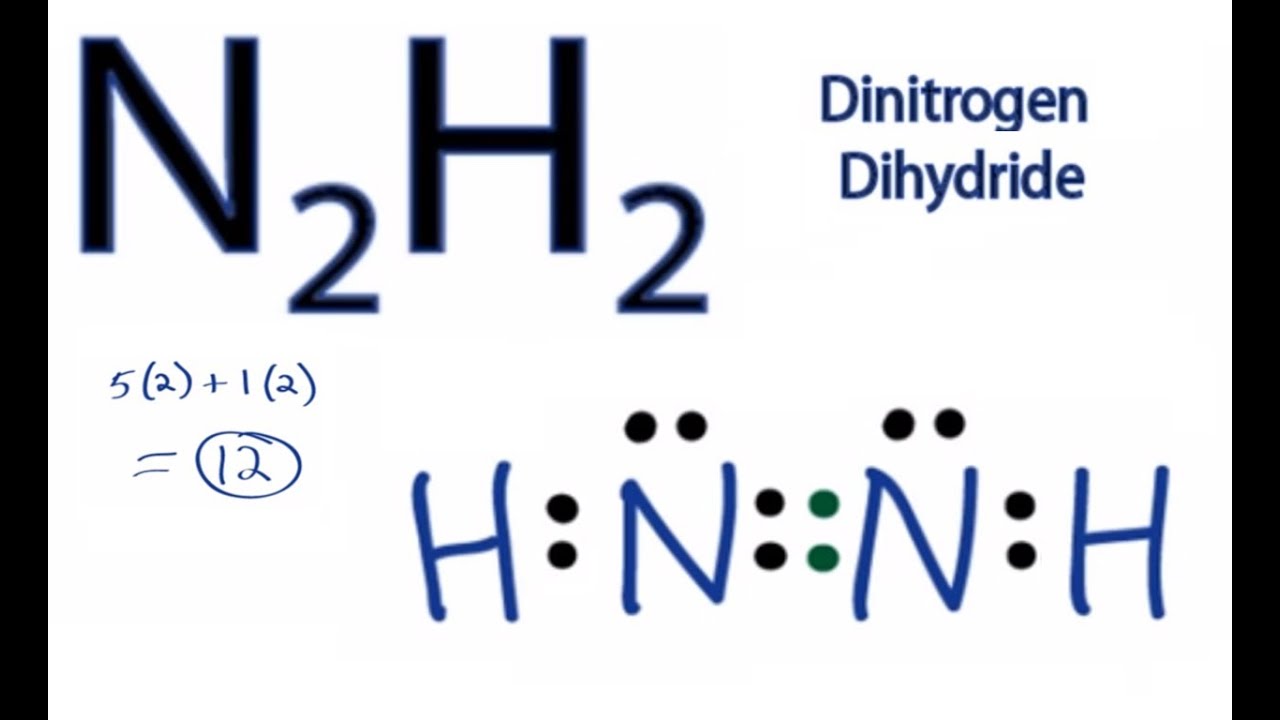
N2h2 Lewis Structure How To Draw The Dot Structure For N2h4 Chemical Bonding

Nh2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Draw The Lewis Structures Of N2h4 N2h2 And N2 Draw The Molecules By Placing Atoms On The Grid And Brainly Com

In The Lewis Structures Of N2h2a There Is Clutch Prep

The Lewis Structure Of N2h2 Shows Study Com

Solution The Lewis Structure For N2h2 Hh Clutch Prep

N2h2 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist