Lewis Structure For H2co Polar Or Nonpolar
Use information from step 4 and 5 to draw the lewis structure. Choose an expert and meet online.
The last one is polar and can hydrogen bond because of the OH group.

Lewis structure for h2co polar or nonpolar. This is the lewis structure for ch2cl2. Draw the Lewis structure of formaldehyde H₂CO and then determine if the molecule is polar or nonpolar. Lewis dot structure of H 2 CO.
Question 1 5 Points Draw a Lewis structure for F 2CO in which the central C atom obeys the octet rule and answer the questions based on your drawing. H2CO Yes or No of e groups on central atom Bonding Non Total Bonding Formal Charges. Quiz your students on Lewis Structure For IBr3 Molecular Geometry Bond Angle Hybridization Polar or Nonpolar using our fun classroom quiz game Quizalize and personalize your teaching.
Beauty Sexy Sensual. CH3NHCH3 -C- N H H 1 H. H2CO C is the central atom.
Question 2 5 Points Draw a Lewis structure for PO 4. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. CCl 2 F 2.
Calculate the total valence electrons in the molecule. H2O2 molecular geometry is bent and electron geometry is tetrahedral. Formaldehyde Methanal H2CO is a trigonal planar molecule AX3 geometry 120 degree bond angle.
The central C atom forms 2 single bonds. Watch this video to know our step-by-step process to determine this molecules geometry. H-CC-C I 1 Н.
C2H5OH is polar molecule. O н н Molecular Shape. How to draw the lewis structure.
Formula Lewis Structure Functional group Hybridization around bold atoms Polar or non- polar Major intermolecular force H H CH3NH2 H-C-N. N 2 O i. Freon-2 CF2Cl2 Polar or Nonpolar based on characteristics CF2Cl2 is a polar molecule and the Fluorine atom closest to the negative side as fluorine is highly electronegative than both chlorine and carbon.
Answer C2Cl4 Tetrachloroethylene is nonPolar What is polar and non-polar. H 2 O m. Hence the molecule is polar.
The number of lone pairs on the central C atom. Carbon is less electronegative than chlorine so itll go on the inside and hydrogens always go on the outside. The polarity of the H2CO relies not only on electronegativities of carbon and oxygen solely.
There is a total of 4 bonded pairs and 3 lone pairs present in the CH3F lewis dot structure. 3- if covelent bond is formed between same non-metal atoms are non-polar covelent bond. CCl 2 F 2 d.
Dont worry we get you. Here is CH3Cl as Chlorine has more electronegativity than all the other atoms it has a partial negative charge and the hydrogen atoms have partial positive charges. Draw Lewis structures name shapes and indicate polar or non-polar for the following molecules.
Choose the best lewis structure for ch2cl2. H2CO CE C2H5CO2H HH o. As a result an overall dipole exists along the CO bond and the molecule.
Total number of valence e. Here is its Lewis Structure and VSEPR shapeCheck me out. Instead the molecule is polar mainly due to the combination of its geometry and.
H 0-H HH H C -H. F atom attracts the electrons from central carbon as a result formation of partial positive charge on carbon and negative charge on fluorine and the same in case of carbon-chlorine. H2co Lewis Structure Polar Or Nonpolar Dowload Anime.
A step-by-step explanation of how to draw the H2CO Lewis Structure Formaldehyde. Write Lewis structure and predict whether the following is polar or nonpolar. Carbon goes in the centreMake sure carbon and oxygen get 8 electrons to fulfil octet rule.
Formaldehyde H2CO is the simpiliest of a class of functional groups call. Being the least electronegative carbon is the central atom in CH3F lewiss structure. CH 2 O f.
Calculate the total valence electrons in the molecule. Hydrogen peroxide is polar in nature and has a non-planar structure. C Electronic Shape.
CF 2 H 2 e. Is H2CO Polar of Non-Polar. To draw the lewis dot structure of CH3F follows some simple steps.
Formaldehyde Were you searching for a video to learn the polarity of H2CO or Formaldehyde molecules. CH3F lewiss structure contains one carbon attached with three hydrogen atoms and one fluorine atom. AeN for Bonds indicate polar or nonpolar.
Is chloromethane polar or nonpolar. NCl 3 trigonal pyramidal polar c. Alternatively a dot method can be used to draw the lewis structure.
CH 4 tetrahedral non-polar b. The bond angle of H2O2 in the gas phase is 948º and in the solid phase it is 1019º. As Daniel correctly stated earlier this molecule is polar but the answer is not that simple.
The total lone pair present in the H2O2 lewis dot structure is 4.

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 In 2021 Lewis Octet Rule Noble Gas

Is H2co Polar Of Non Polar Youtube
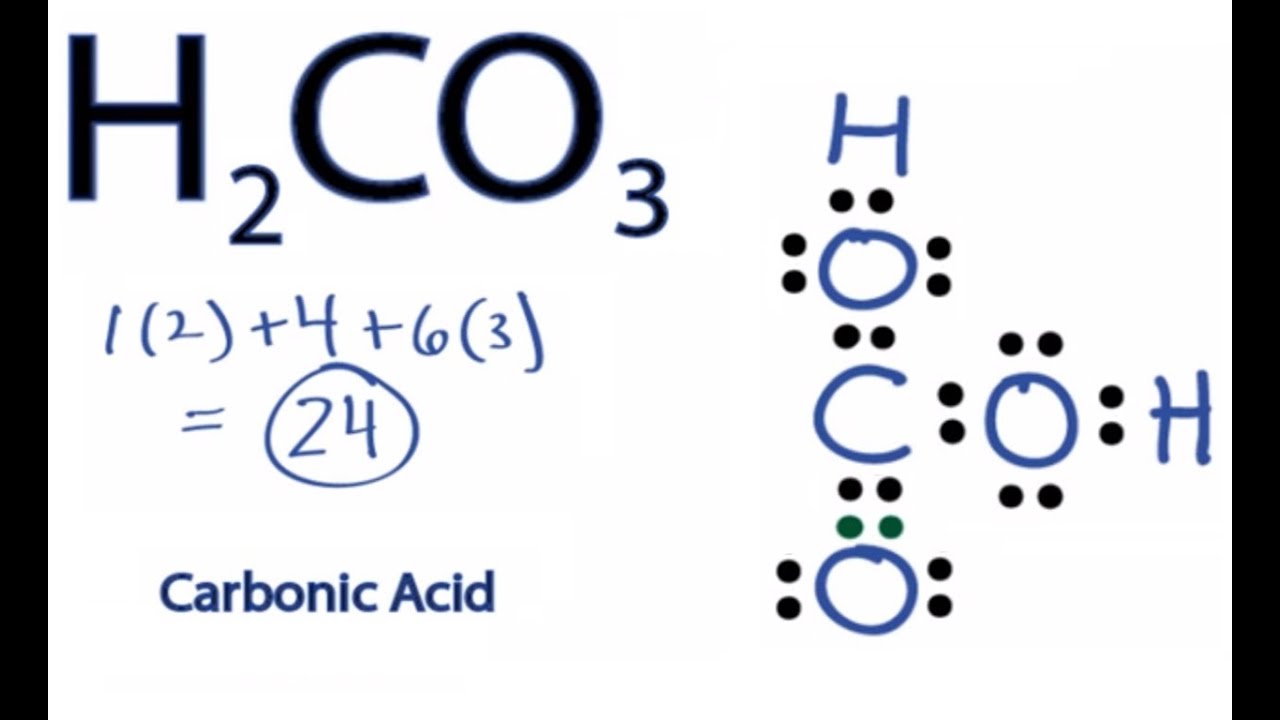
H2co3 Lewis Structure How To Draw The Lewis Structure For Carbonic Acid Youtube
Ch2cl2 Lewis Structure Molecular Geometry Polarity Dichloromethane
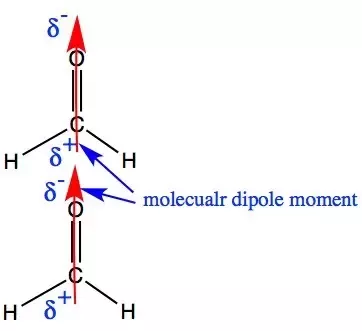
Is H2co A Polar Or Non Polar Molecule Quora

How To Draw The Lewis Dot Structure For Ch2o Formaldehyde Youtube
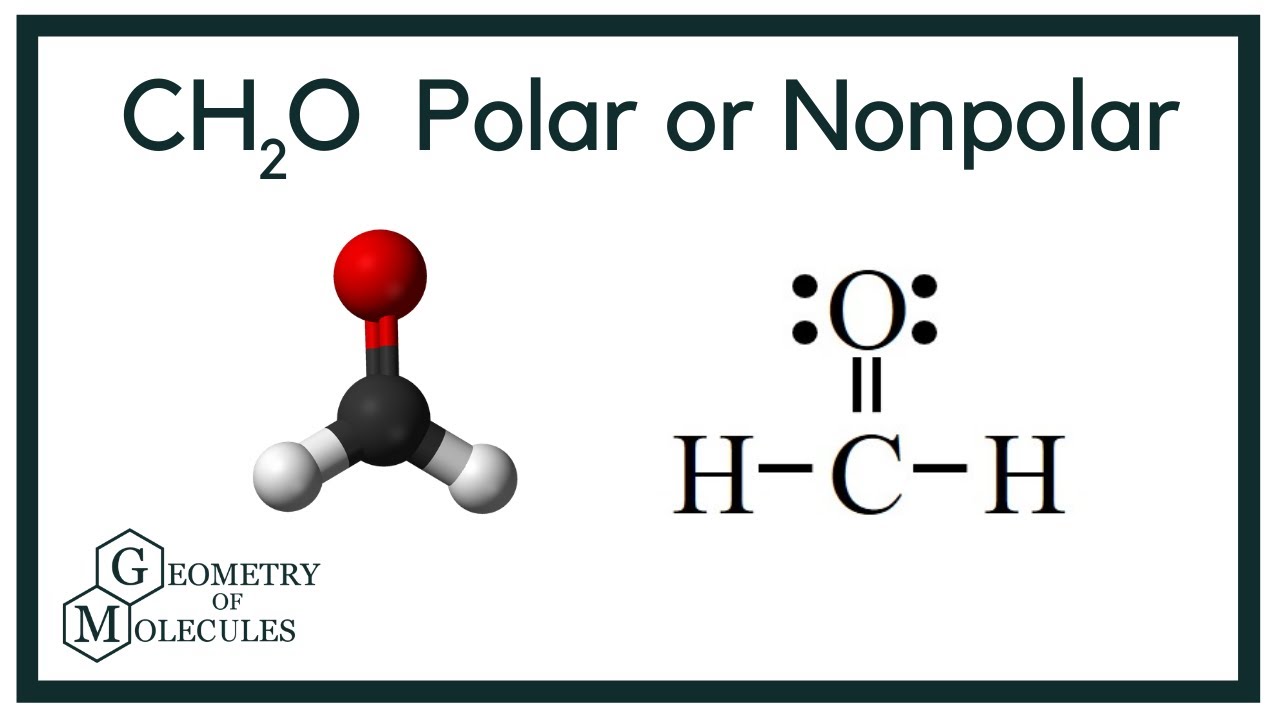
Ch2o Molecular Geometry Polarity Bond Angle Shape Geometry Of Molecules

Is O3 Polar Or Non Polar Ozone In 2021 Ozone Chemical Formula Polar

Is Bf3 Polar Or Non Polar Boron Trifluoride In 2021 Boron Atom Molecules Chemical Formula
Lewis Structures And Hybridization Quiz Quizzma
Bf3 Lewis Structure Molecular Geometry Hybridization And Polarity
Is H2co A Polar Or Non Polar Molecule Quora

How Do I Use The Lewis Structure Drawing Tool 101edu

Is Bh3 Polar Or Non Polar Boron Trihydride Youtube

H2co Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Ch4o Lewis Structure How To Draw The Lewis Structure For Ch4o Youtube

H2co Lewis Structure How To Draw The Electron Dot Structure For H2co

