How To Calculate Formal Charge Of Co2
Where V Number of valence electrons. Formal charge of C 4 - 0 - 82 0.

Co2 Lewis Structure And Molecular Geometry What S Insight
A formal charge can be defined as the electrical charge of an atom within a molecule and calculated by determining the total number of valence electrons minus half the total electrons located in a shared bond minus the number of electrons not in the molecule.

How to calculate formal charge of co2. Oxygen double 0. It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. Two other possibilities are carbon radicals and carbenes both of which have a formal charge of zero.
Formal charge H 1 12 2 0 0 This applies to each hydrogen. Formal charge C 4 12 6 2 4 3 2 -1. One can calculate the formal charges for any given atom with the help of the following formula.
Formal charge of C 4 - 2 - 62 - 1 Formal charge of O 6 - 2 - 62 1 2. In the Lewis-dot structure the valance electrons are shown by dot. Identify and recognize the bonding patterns for atoms of carbon hydrogen oxygen nitrogen and the halogens that have a.
Lewis structures also show how atoms in the molecule are bonded. P and F Si and F P and Cl Si and Cl. The total charge of Carbon Dioxide is 0 since the oxidation numbers of Carbon and Oxygen are 4 and -2 respectively.
Formal charge FC is given by the formula. Since carbon has 4 valence electrons its formal charge will be zero. These hydrogens are all zero.
FC Valence electrons Nonbonding electrons- Bonding electrons2. Oxygen single 1. Subtract the number of electrons in the circle from the group number of the element the Roman numeral from the older system of group numbering NOT the IUPAC 1-18 system to determine the formal charge.
Summing Up Formal Charge. Formal charge Valence electrons - Nonbonding electrons - Bonding electrons2 Let the Lewis structure of the compound aid you. It is important to keep in mind that formal charges are just that formal in the sense that this system is a formalism.
These charges help in knowing if the given structure of the molecule is stable or not. Mathematically the formal charge of an atom in a molecule is calculated as the number of valence electrons minus the number of electrons not bound on a molecule lone-pair electrons minus half of. Required fields are marked What Is The Formal Charge On Each Atom In Co2.
Total formal charge 0. In the carbonate ion the carbon atom is bonded with a double bond to an oxygen atom and with single bonds to two oxygen atoms. The formal charge on the carbon atom of carbon monoxide in its major resonance form triple bonded with oxygen is -1.
The formal charge on CO₂ is Zero 0 Explanation. Every element in the compound has its own formal charge. The given molecule is.
Formal charge is the individual electric charges on the atoms in a given polyatomic molecule. The carbonate ion CO 3 2- has an overall charge of -2. Formal Charges calculate the formal charge of an atom in an organic molecule or ion.
First we have to determine the Lewis-dot structure of. Carbon single bonded to one oxygen and double bonded to another. Molecule with 16 total valence electrons.
Calculate The Formal Charge Of Atoms In CO CO2. Carbon double bonded to both oxygen atoms carbon 0 oxygens 0 total formal charge 0. The formal charge on each of the atoms can be calculated as follows.
A quick way to calculate formal charge is to count subtract the sum of the number of lone electrons and bonds by the number of valence electrons. In order to calculate the formal charges for CO2 well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding elec. In order to calculate the formal charges for CO3 2- well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding e.
The formal charges computed for the remaining atoms in this Lewis structure of carbon dioxide are shown below. The formal charge on an atom can be calculated using the following mathematical equation. These hydrogens are all zero.

Calculate The Formal Charge On Co2 Brainly In

Co2 Lewis Structure Easy Hard Science

Lewis Structures Formal Charge And Co2 Youtube

Co2 Lewis Structure Easy Hard Science
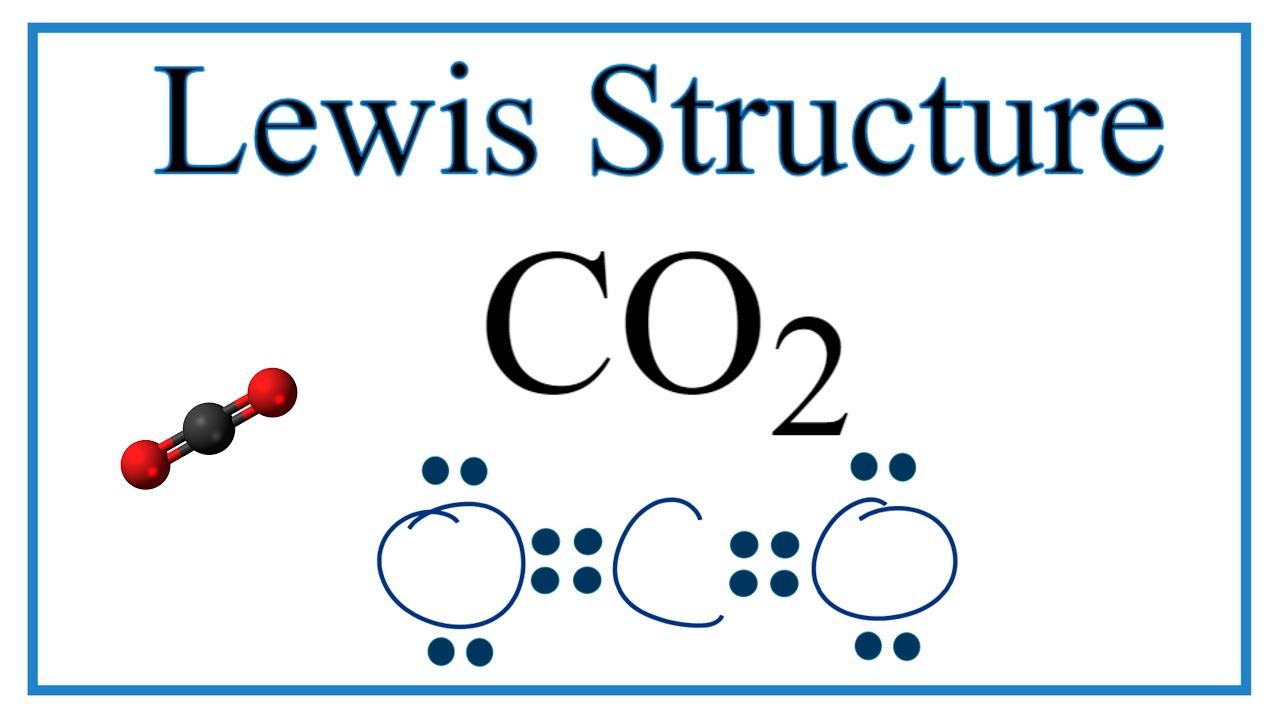
How To Draw The Lewis Dot Structure For Co2 Carbon Dioxide Youtube

Practice Calculating Formal Charge With This Chemistry Sample Problem Chemistry Octet Rule Chemistry Notes

Co2 Lewis Structure And Molecular Geometry What S Insight

Is Co2 Ionic Or Covalent Techiescientist

Molecular Orbital Diagram For Polyatomic Molecules Beh2 H2o Co2 Nh3 Sf6 Molecular Molecules Chemistry

Number Of Lone Pairs And Bonding Pairs For Co2 Carbon Dioxide Youtube

Lewis Structure Practice Worksheet 4 Stepsa Ch4 Lewis Structure In 2020 Practices Worksheets Graphing Linear Equations Chemistry Worksheets

Lewis Dot Structure For Hydrogen H Lewi Electron Configuration Chemistry Worksheets Chemical Equation

Co2 Lewis Structure Molecular Geometry And Hybridization Molecular Geometry Molecular Lewis

Lewis Structure Co2 Plus Dipoles Shape Angles And Formal Charge Youtube

In Chemistry Drawing Lewis Dot Structures Can Be Challenging But They Provide A Wealth Of Information About The Molecules Th Chemistry In Pictures

Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube
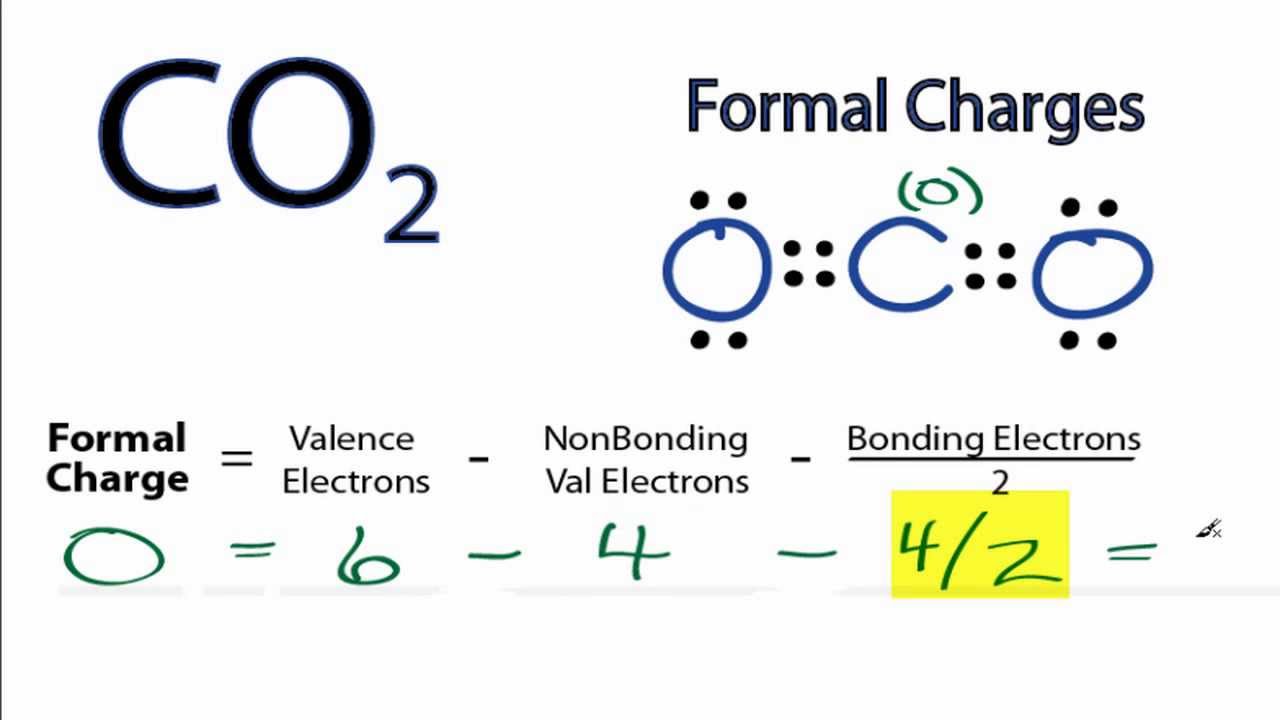
Calculating Co2 Formal Charges Calculating Formal Charges For Co2 Youtube

Resonance Structures For Co2 Carbon Dioxide Youtube
Co2 Lewis Structure Molecular Geometry And Hybridization