Xef2o Lewis Structure Formal Charge 0
Now lets look at the cationic form of methanol CH 3 OH 2. Asked by RQ on September 17 2014 chemistry Draw the lewis structure for.

Xef2o Lewis Structure Xenon Oxytetrafluoride Youtube
Who are the experts.

Xef2o lewis structure formal charge 0. Show all lone pairs. The fluorines are not attached to the xenon with a covalent bond. In chemistry the formal charge is used to find out the charge acquired by an atom in a molecule.
And of course ONE of the oxygen bears a FORMAL. The molecule H3PO3 one P-H bond does not obey the octet rule. Remember that Xenon can have more than 8 valence electrons.
The nitrogen is quaternized and bears a FORMAL POSITIVE CHARGE. Carbonate ion is used frequently in chemistry and worth spending tim. We think so because all the atoms in f have a formal charge of zero.
Include ions pairs and charges. Youll want to calculate the formal charges on each atom to make sure you have the best Lewis structure for XeO 2 F 2. When you write Lewis structures you should include formal charges next to each atom with a formal charge that isnt 0.
A stepbystep explanation of how to draw the co32 lewis structure carbonate. Draw a lewis structure for a resonance form of ClO2- showing the lowest possible formal charges and give the oxidation numbers of the atoms Chemistry Give the molecular orbital description eg. The formal charge on the Fluorine F is 0.
Now if we look at Lewis structures e and f with formal charges we can predict with reason that structure e should be stable. The bottom line is that this structure does not violate the octet rule. Sigmasp3-sp3 for each unique bond in the following structure.
The Lewis structure for XeF2 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. There is no valance to this molecule so you want to satisfy the formal charge to be 0. Experts are tested by Chegg as specialists in their subject area.
Postby Kyra Dingle 1B Sat Dec 09 2017 654 am. 4 - 0 - 4 0. It is helpful if you.
In doing so you can see that Xe has 5 electron densities with 4 bonded pairs and one lone pair. It is calculated using the number of valence electrons bonded electrons and. Since the negative charge should reside on the most electronegative atom if follows that Lewis structure.
If you calculate the formal charge of the final structure you will find that all atoms have zero formal charge. XeO2F2 is polar and it helps to identify this through drawing out the lewis structure. Draw its Lewis structure and state the type of octet-rule exception.
Formal Charge Number of Valence electrons - number of dots - number of lines. But check the formal charges -- its probably not the be. B and thanks for watching.
A stepbystep explanation of how to draw the po4 3 lewis structure phosphate ion. Nitric acid is an interesting customer in terms of Lewis structure given that TWO of the FIVE constituent atoms bear FORMAL charges. XeF 2 is dsp 3 hybridized and contains 3 lone pair and 2 bonding pairs of valence electrons around the Xenon.
The formal charges closer to zero mean a more plausible or likely Lewis structure. This is what we want. So the formal charge on carbon is zero.
This one is a bit tough since the first Lewis structure you generate will seem like the right one. Make a sketch of IF2-. Usually you circle the charge so its clear This can also help you tell which Lewis structures are good.
They are simply attracted to it in a charge-transfer bond. 7 - 6 - 1. However in structure f notice that N has a formal charge of 1 while C has a formal charge of 1- but N is more electronegative than carbon.
Try to draw the XeF 2 Lewis structure before watching the video. Write a Lewis structure and identify the octet-rule exception for 1. We review their content and use your feedback to keep the quality high.
Draw the lewis structure of CP. For each of the hydrogens in methanol we also get a formal charge of zero. The single lone pair gives the compound a seesaw shape which then would be polar.
Formal charge electrons in ground state - free electrons - 12 bonding electrons. That makes this the most plausible or likely Lewis structure for Cl3PO. This chemistry video tutorial explains how to draw lewis structures of molecules and the dot diagram polyatomic ions.
So weve used all 32 valence electrons each of the atoms have octets and our formal charges are 0. SiO3 -2 CNO- TeO4 -2 F2PPCl2 thanks. Asked by patricio on November 4 2011 chemistry Given that S is the central atom draw a Lewis structure of OSF4 in which the formal charges of all atoms are zero.
Formal charge on hydrogen 1 valence electron on isolated atom - 0 nonbonding electrons - ½ x 2 bonding electrons 1 - 0 - 1 0. What is the lewis structure of XeF2O.

Xef2o Lewis Structure How To Draw The Lewis Structure For Xef2o Youtube
Why Is The Lewis Structure Of So2 Not Similar To 03 Quora

Answer Draw Lewis Structures For The Foll Clutch Prep

Draw Lewis Structure For Xef2o

What Is The Lewis Structure For Cho2 Quora
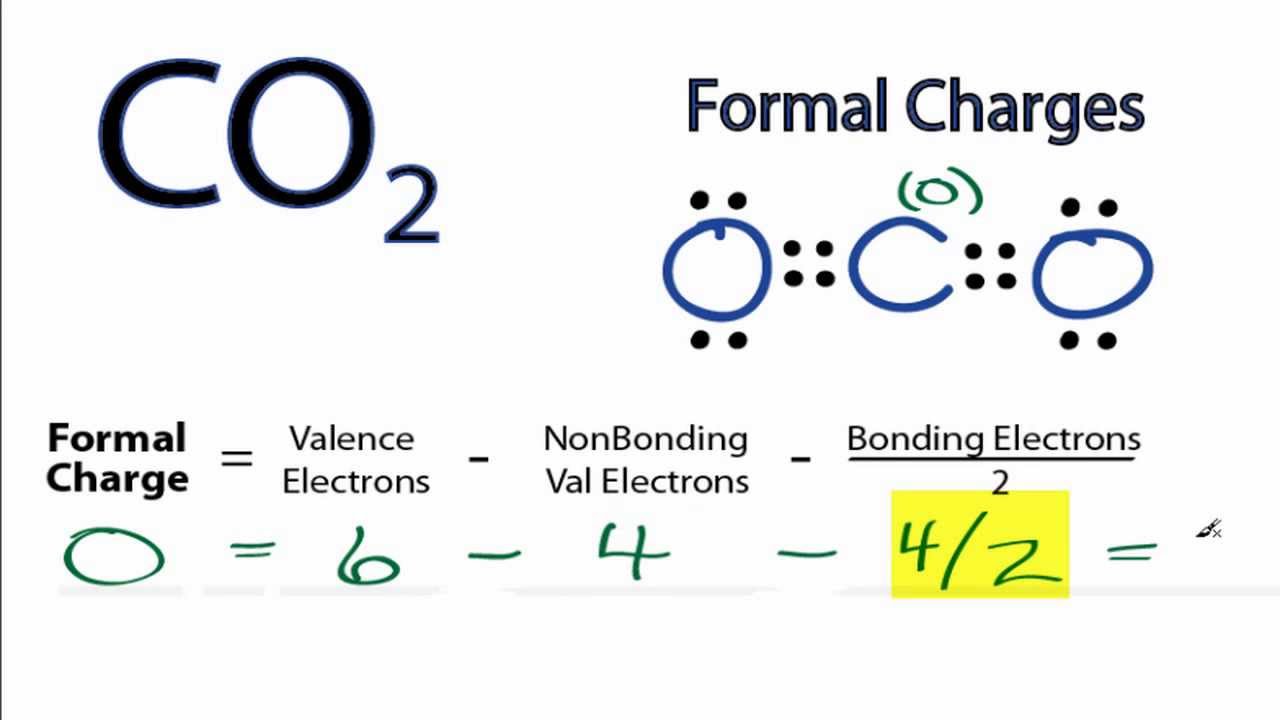
Calculating Co2 Formal Charges Calculating Formal Charges For Co2 Youtube
How To Draw An Xef2o Lewis Structure Quora
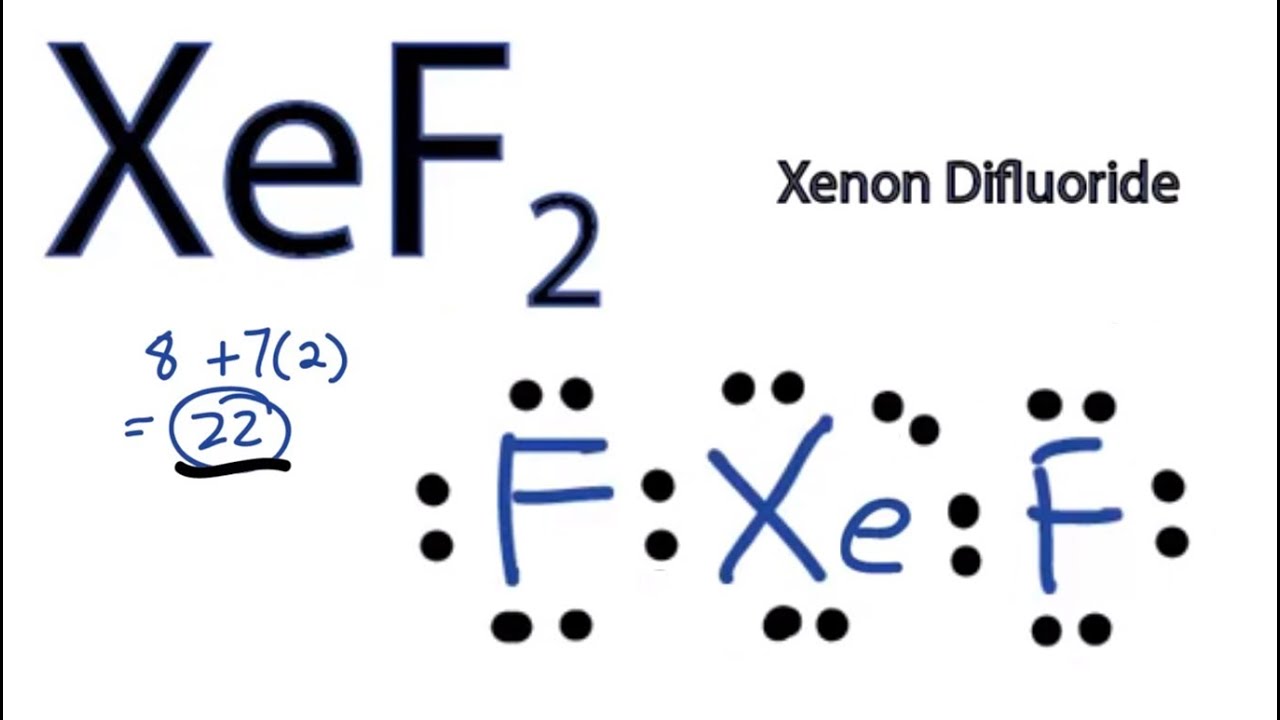
Xef2 Lewis Structure How To Draw The Lewis Structure For Xef2 Youtube

On Feedback Xef Should Have A Total Of 8 2 7 Chegg Com
How To Draw Lewis Structure For Xef2 Drawing Easy

Xef2o Lewis Structure Xenon Oxytetrafluoride Youtube

How To Draw An Xef2o Lewis Structure Quora

Draw Lewis Structure For Xef2o

Xef2o Lewis Structure How To Draw The Lewis Structure For Xef2o Youtube
How To Draw An Xef2o Lewis Structure Quora

Determining Formal Charge Carbon Monoxide 001 Youtube
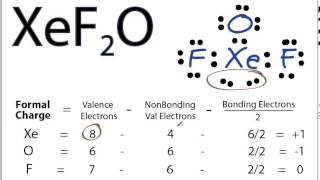
Xef2o Lewis Structure How To Draw The Lewis Structure For Xef2o Youtube

Xeo2f2 Lewis Structure How To Draw The Lewis Structure For Xeo2f2 Youtube
