Lewis Diagram Of C2h4
There are only single bond between carbon atom and hydrogen atom because hydrogen caannot keep more than two electrons in its last shell. Hence total shared pairs of electrons in the Lewis dot structure of C2H4 is 12.
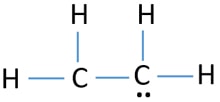
Ethene C2h4 Lewis Structure Hybridization
Drawing the Lewis dot structure for C2H4 ethene and answer the questions below.
Lewis diagram of c2h4. Ethylene C2H4 has sp2 hybridization and its bond angle is between 1166 to 1217. In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair. Lewis Dot Structure for C2H4 6 of 6 Watch the video of Dr.
Hydrogen is the least electronegative element here. Subtract step 1 total from step 2. As per the Ethene Lewis dot structure Four CH sigma bonds are present and one CC double bond1 sigma 1 pie bond.
H-----C---H SHOW WORK This question is worth a total of 6 points. Drawing the Lewis structure for C 2 H 4 named ethene requires the use of a double bond. Two dots between two atoms represent a covalent bond.
Draw the Lewis structure for C2H4. A step-by-step explanation of how to draw the C3H4 Lewis Dot StructureThere are three different ways probably more to draw the C3H4 Lewis structure. The ethylene is a covalent bond and lewis base.
Arrangement of atoms shown below dashed lines show connections between atoms. The molecular geometry of C2H4 is trigonal planar above are the explanation of its Lewis structure. We place two valence electrons between each atom as shown in the figure.
Gives you bonding e-. Find number of bonds by diving the number in step 3 by 2 because each bond is made of 2 e- 12e-2 6 bond pairs. However in Hydrocarbons we always place the Carbon atoms in the center as shown in the figure.
1 point for the correct selections assessed when you answer and 5 points for the Lewis structure on your work assessed when I review. This means that the carbon atoms share 4 electrons. Note that the C2H4 Lewis dot structure involves.
Answered by Expert. C2H4 Lewis Structure Ethylene Welcome to Geometry of Molecules and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure of C2H4 molecule. The carbon atom consists of 6 electrons and hydrogen has 1 electron.
Drawing the Lewis dot structure for C2H4 ethene and answer the questions below. Start by forming covalent bonds between the Carbon and Hydrogen atoms. For C 2 H 4 you have a total of 12 total valence electrons.
Answered by ExpertThe Lewis structure for C2H4 consists of two C symbols connected by two lines. In fact Lewis structures are very important for predicting geometry polarity and reactivity of inorganic compounds. Each C symbol is connected to two H symbols with a single line for each.
Find the number of nonbonding lone pairs e-. It is a chemical formula for Ethylene or Ethene. According to the VSEPR chart the shape of the ethene molecule is trigonal planar.
In a double bond two pairs of valence electrons are shared for a total of four valence electrons. Therefore there cannot be more than one stable resonance structure for C 2 H 4. Note that the C 2 H 4 Lewis dot structure involves sharing more than one pair of electrons.
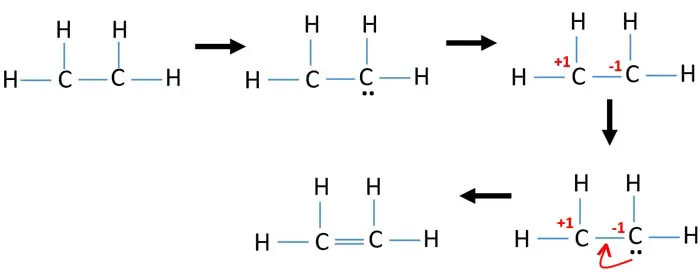
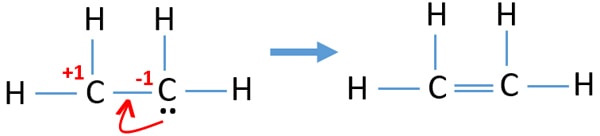
When we look at the molecules of C2H4 it has 2 CH molecules and 4 H molecules. In the lewis structure of C 2 H 4 there are only four C-H bonds one CC bond and no lone pairs on last shells. During the formation of CH 2 CH 2 the electronic configuration of carbon in its ground state 1s 2 2s 2 2p 1 2p 1 will change to an excited state and change to 1s 2 2s 1 2px 1 2py 1 2pz 1.
We have 12 available valence electrons. On Lewis Dot Diagram For C2h4. C represents carbon atoms and H represents hydrogen atoms.
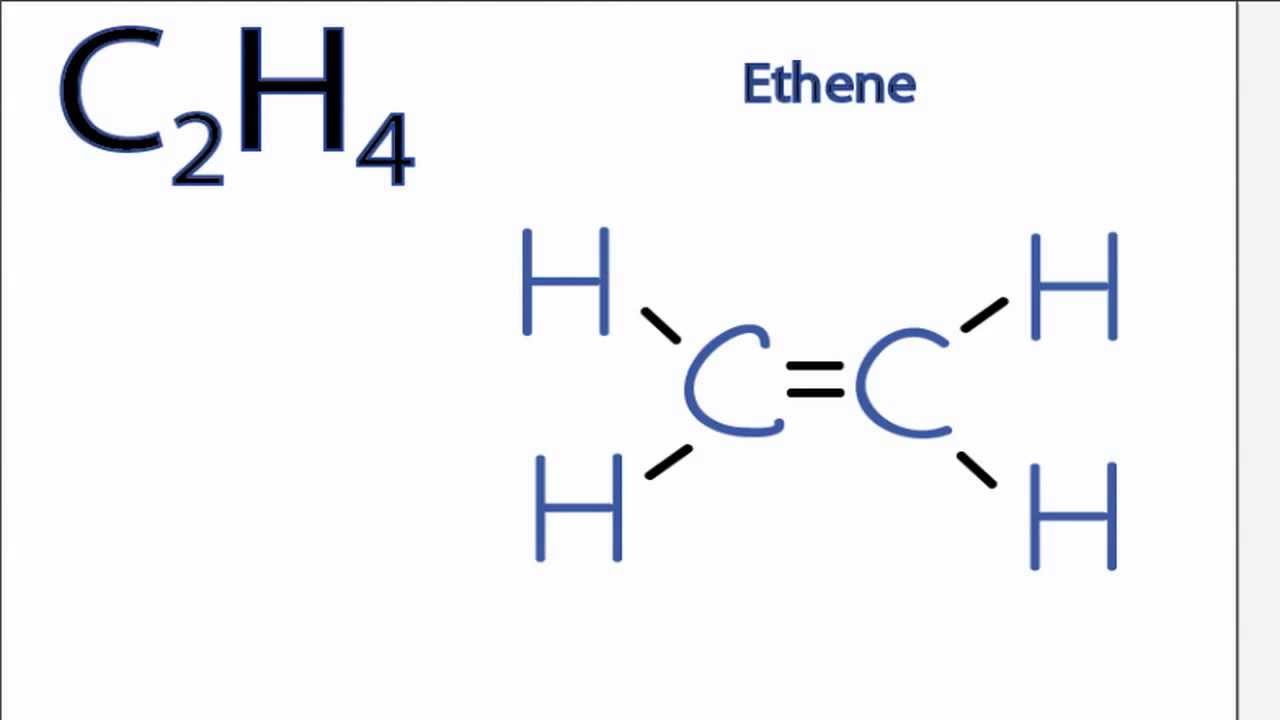
Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence. The Lewis structure of C2 H4 also known as ethene has two carbons with a double bond between them. Let us look at how the hybridization of ethene ethylene occurs.
Drawing the Lewis Structure for C 2 H 4. C2H4 Lewis Structure. It means four CH bond has 8 shared pairs of electrons and CC bond has 4 shared pairs of electrons.
Lewis structure also called electron-dot structure is a structural formula in which electrons are represented by dots. How many shared pairs of electrons are in the lewis dot structure of C2H4. Watch the video of Dr.
There are two triangles overlapping each other as we can see in the diagram. D The Lewis electron-dot diagram for C2H4 is shown below in the box on the left.

How To Calculate The Formal Charges For C2h4 Ethene Youtube

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Molecular Geometry Shape And Bond Angles Youtube
Lewis Structure Of C2h4 Biochemhelp

Write Lewis Structure Of C2h4 Brainly In
Is C2h4 Polar Or Nonpolar Youtube

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Ethene C2h4 Lewis Structure Hybridization
Ethene C2h4 Lewis Structure Hybridization
Lewis Electron Dot Structures Ck 12 Foundation

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar

Draw The Electron Dot Structure Of Ethene C2h4 Brainly In

C2h4 Lewis Structure C2h4 Lewis Structure Molecular Geometry

Draw The Lewis Structure For The C2h4 Ske Clutch Prep

