Cocl2 Lewis Structure Shape
It is an inorganic compound that comprises Cobalt and Chlorine atoms. Phosgene is a colorless gaseous compound known as carbonyl chloride and has a molecular weight of 9892 grammol.
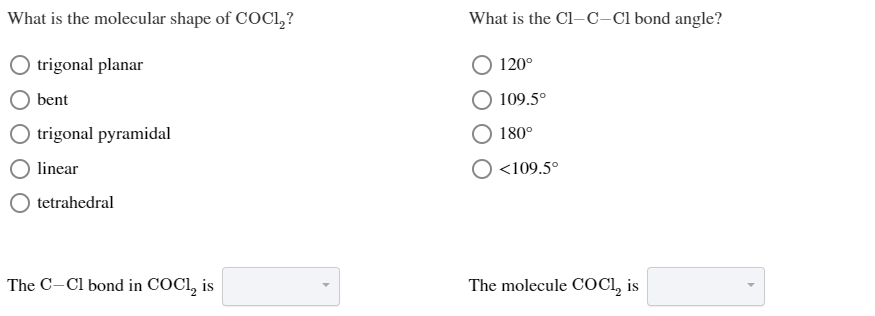
Answered What Is The Molecular Shape Of Coc12 Bartleby
CS2 Total of Valence Electrons.

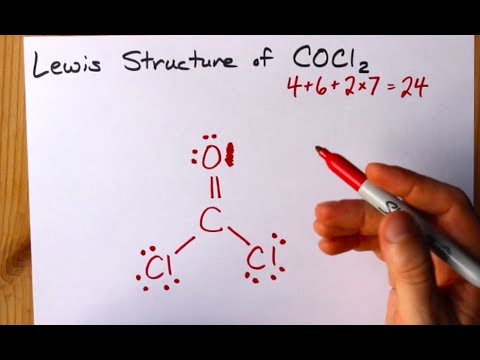
Cocl2 lewis structure shape. Alternatively a dot method can be used to draw the lewis structure of COCl 2. C4O6Cl2x714 Total24 Put carbon in the center. Starting with its Lewis structure the C2Cl2 molecule has a total of 22 valence electrons 4 from each of the two carbon atoms and 7 from each of the two chlorine atoms.
COCl2 is a chemical compound known by the name phosgene. What is the molecular shape of COCl2. Jan 12 2015.
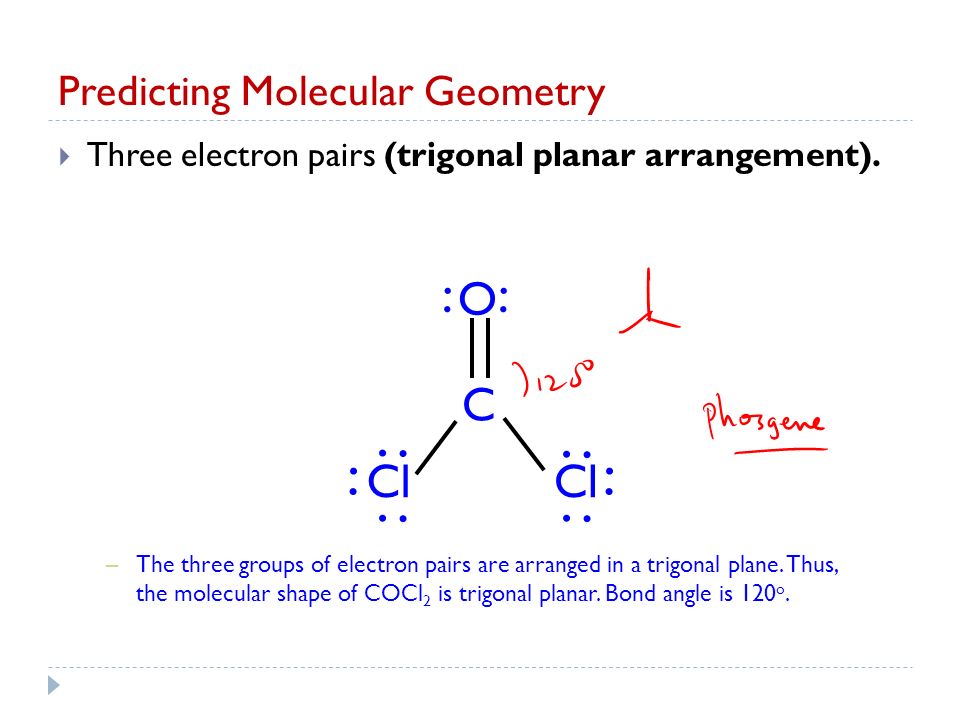
The COCl2 molecule has 3 areas of electron repulsion around the central C atom so the shape is trigonal planar. Thus the molecular shape of COCl2 is trigonal planar. The Lewis structure of the COCl2 molecule shows that there is a double bond between the O and the central C atom.
You have two double bonds or two electron groups about the carbon atom. Arrangement - AX3 with 3 bonding pairs no lone pairs on the central atom. Lewis Structure show all resonance structures if applicable Molecular Shape.
Draw the Lewis structure for COCl2 including lone pairs. It is readily soluble in water alcohol and acetone. I hope this helps.
The Lewis structure of the CoCl2 molecule shows that there is a double bond between the O and the central C atom. The molecular geometry of carbonyl fluoride is ideal trigonal planar with a bond angle of 120. Moreover there exist many lone pairs which do not alter the molecular geometry but make the molecule polar.
I was wondering if COCl2 was polar or nonpolar. A double bond represents a slightly larger electron domain which disrupts the ideal 120 Cl-C. Any polar bonds in the molecule.
Both CCl bonds are polar due to the difference in electronegativity of C and Cl. The chemical formula CoCl2 represents Cobalt II Chloride. Yes No Molecular Symmetry.
COCl2 Lewis Structure Molecular Geometry Hybridization and Polarity. In the cocl2 lewis structure carbon is less electron electronegative than oxygen and goes in the center of the lewis structure note that hydrogen atoms always go on the outside. The bond angle is 180o.
Whereas the hybridization type again turns out to be the ideal shape which is sp2 vastly followed by all the trigonal planar shaped molecules. Is the CCl bond in COCl2 polar or. Thus according to the VSEPR model the bonds are arranged linearly and the molecular shape of carbon dioxide is linear.
Both CCl bonds are polar due to the difference in electronegativity of C and Cl. The three groups of electron pairs are arranged in a trigonal plane. Get the detailed answer.
Calculate the total valence electrons in COCl 2 molecule. In the lewis structure for cocl2 there are a total of 24 valence electrons. Two electron pairs linear arrangement.
3-D Model Sketch Bond Angles Lewis Structure show all resonance structures if applicable Molecular Shape. CoCl2 Lewis Structure Molecular Structure Hybridization Bond Angle and Shape. 3-D Model Sketch Bond Angles Lewis Structure show all resonance structures if applicable.
Thus its Lewis structure must account for. COCl2 Total of Valence Electrons. The COCl2 molecule has 3 areas of electron repulsion around the central C atom so the shape is trigonal planar.
CoCl2 is a crystalline solid that is sky-blue in color. The molecular shape of the C2Cl2 molecule is linear. A double bond represents a slightly larger electron domain which disrupts the ideal 120 CI-C-Cl bond angle making it smaller.
It is non-flammable in nature and bears a suffocating odor. The L View the full answer. Lewis Structure show all resonance structures if applicable Molecular Shape.
Lewis dot structure of CO Cl 2. Yes No Molecular Polarity. I drew its Lewis structure and got a trigonal planar shape with a double bond on the oxygen and I would normally think it was nonpolar because of the symmetrical shape however I am aware that O has a higher electronegativity than Cl so maybe the net dipole moment might be pointing towards O which would make it polar but Im not sure.
Any polar bonds in the molecule.

Cocl2 Phosgene Molecular Geometry Bond Angles And Electron Geometry Youtube
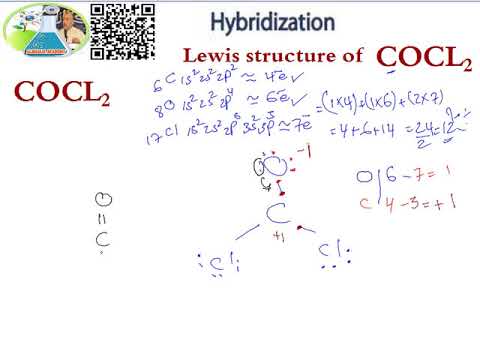
Lewis Structure Hybridization Cocl2 Youtube

What Is The Shape Molecular Geometry Of Cocl2 A Trigonal Clutch Prep
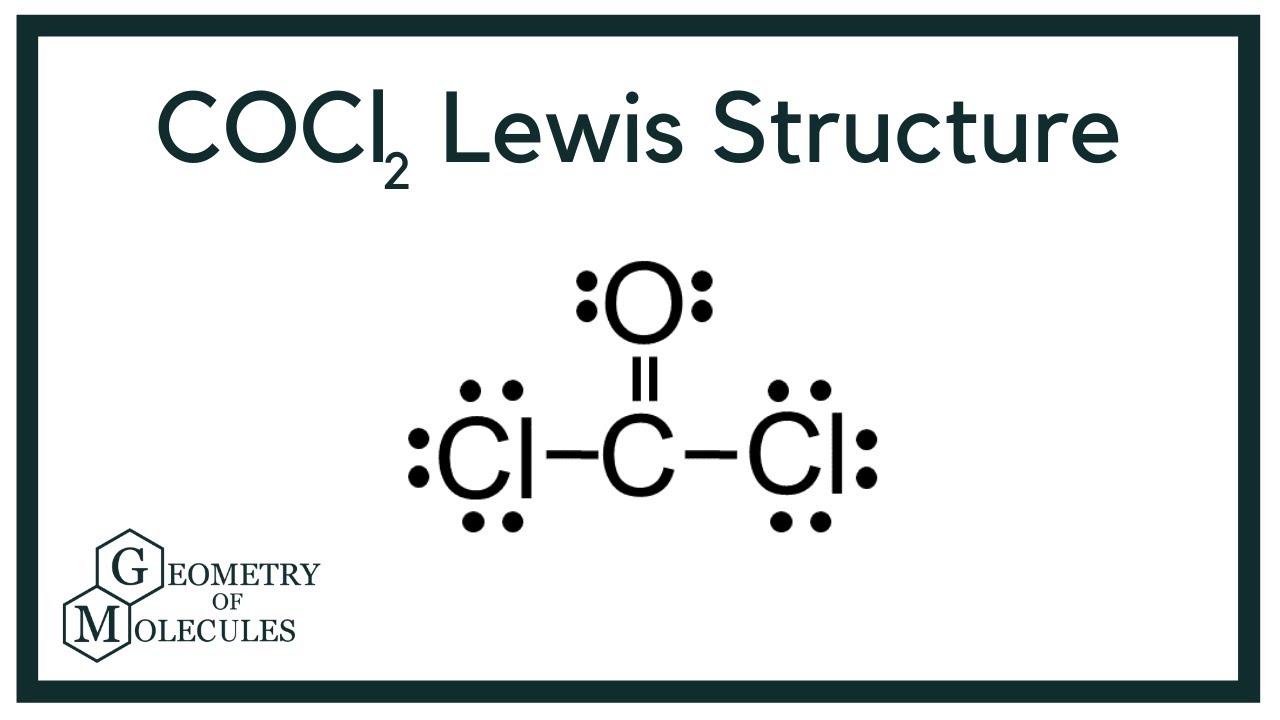
Cocl2 Lewis Structure Phosgene Youtube

Cocl2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
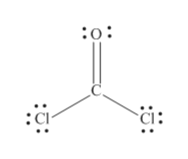
Answered What Is The Molecular Shape Of Coc12 Bartleby

Cocl2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
Carvone Bucky Ball Molecular Geometry Chapter 8 Part Ppt Video Online Download

Cocl2 Lewis Structure How To Draw The Lewis Structure For Cocl2 Youtube

What Is The Molecular Shape Of Cocl2 Quora
Cocl2 Phosgene Molecular Geometry Bond Angles And Electron Geometry Youtube
How To Draw The Lewis Structure Of Cocl2 Dichloromethanal Phosgene Youtube

Chemical Bonding Ii Molecular Geometry And Hybridization Of Atomic Orbitals Chapter Ppt Download

How To Draw The Lewis Structure Of Cocl2 Dichloromethanal Phosgene Youtube

What Is The Shape Molecular Geometry Of Cocl2 A Trigonal Clutch Prep

Cocl2 Lewis Structure How To Draw The Lewis Structure For Cocl2 Youtube
Vsepr Shape Of Cocl2 Biochemhelp