Best Lewis Structure For H2co3
Then determine the maximum number of equivalent resonance structures for each species. This is the best Lewis structure because it does not leave formal charges on any individual atoms.

How To Draw A Lewis Structure Lewis Chemistry Draw
Why Is My Lewis Structure For HCO3- Incorrect.

Best lewis structure for h2co3. Put carbon in the center and arrange hydrogen and oxygen atoms on the sidesArrange electrons until both carbon and oxygen get 8 electrons. Draw out the Lewis structures for H2CO3 HCO3- and CO32-. Only count the best structures.
H2SO3 Lewis structure Report. If the molecule contains hydrogen atoms they are attached to oxygen atoms. Gaseous CO2 once dissolved combines with water to form carbonic acid dissolved CO2 H2O - H2CO3 and dissociates reversibly to yield hydrogen and bicarbonate ions H2CO3 H HCO3-.
When we have an H or H2 in front of a polyatomic molecule like CO. Draw out the Lewis structures for H 2CO3 HCO3- and CO32-. Draw the best Lewis structure for H2CO3 and calculate the formal charge on carbon see more.
Writing the Name for. Asked Jan 27 2020 in Chemistry by SurajKumar 662k points Draw Lewis structures for H 2 CO 3 SF 6 PF 5 IF 7 and CS 2. Write Lewis structures for CO 3 2- HCO 3- and H 2 CO 3.
Then determine the maximum number of equivalent resonance structures for each species. Then determine the maximum number of equivalent resonance structures fo. When acid is added to an aqueous solution containing carbonate or bicarbonate ions carbon dioxide gas is formed.
A step-by-step explanation of how to draw the H2SO3 Lewis Structure Sulfurous acid. Note that carbon is the central atom in all three cases. After finishing the lewis structure of CO 3 2- there should be a -2 charge and it should be stabile structure.
How to Draw the Lewis Structure of Bicarbonate HCO3. Total valence electrons concept is used to draw the lewis structure of CO 3 2-. The dominant acid-base buffer system in humans is the carbonic acid H2CO3-bicarbonate HCO3 buffer system.
Lewis electron dot structure of carbonic acid H2CO3. Draw Lewis structures for H2CO3 SF6 PF5 IF7 and CS2. Draw the best Lewis structure for H2CO3 and calculate the formal charge on carbon.
This is a pattern seen with many acids. If playback doesnt begin shortly try restarting your device. Up Next How to.
Day02 3 Lewis Structure. You will learn about these facts in this tutorial. Each oxygen atom has two pairs of free electrons.
Lewis structure of carbonate ion is drawn in this tutorial step by step. How to Draw the Lewis Structure for. There are no lone pairs on carbon atom.
The two hydrogen atoms are attached to each oxygen atom. So well put the Carbon at the center. Lewis Structure for CO 3 2- Carbonate ion.
Draw The Best Lewis Structure For H2CO3 And Calculate The Formal Charge On Carbon. By knowing the Lewis structure we can also predict the three-dimensional geometry of an individual molecule. CO32- Lewis Structure - How to Draw the Lewis Structure.
A step-by-step explanation of how to draw the H2CO3 Lewis Structure Carbonic AcidWhen we have an H or H2 in front of a polyatomic molecule like CO3 SO. A step-by-step explanation of how to draw the H2CO3 Lewis Dot Structure Carbonic AcidFor the H2CO3 structure use the periodic table to find the total numb. The Lewis dot structure of carbonic acid H2CO3 is as follows.
So there are two pairs of dots. Draw the best Lewis structure for H2CO3 and calculate the formal charge on carbon. Prev Question Next Question 0 votes.
In the lewis structure of carbonic acid H 2 CO 3 carbon atom is the center atom and there are two -OH groups. Also there is one double bond between carbon and oxygen atoms. H2CO is the simpliest example of the organic functional group called the Aldehydes.
Only count the best structures. For example A structure with really bad formal charges should not be counted. H 2 CO 3 Carbonic Acid Lewis Structure.
One oxygen atom is bonded with the carbon atom by double covalent bond. Carbonic acid is a molecule which contains one carbon atom three oxygen atom and two hydrogen atom. The pure compound decomposes at temperatures greater than ca.
Two oxygen atoms are attached to a carbon atom by single coolant bonds. When we have an H or H2 in front of. If the molecule contains hydrogen atoms they are attached to oxygen atoms.
We generally say that carbonic acid H 2 CO 3 is unstable. Draw out the Lewis structures for H2CO3 HCO3- and CO32-. Note that carbon is the central atom in all three cases.
Is the octet rule obeyed in these cases. Paper was 100 unique. For example a structure with really bad formal charges should not be countedThe molecule H2CO3 has_____ equivalent Lewis structures.
Use bond energies to estimate ΔE for the reaction in the gas phase H 2 CO 3 CO 2 H 2 O. Get the detailed answer. Is the octet rule obeyed in these cases.

How To Draw A Lewis Structure Lewis Chemistry Draw

Lewis Structures Made Easy Examples And Tricks For Drawing Lewis Dot Diagrams Of Molecules Yo High School Chemistry Teaching Science Organic Chemistry Study
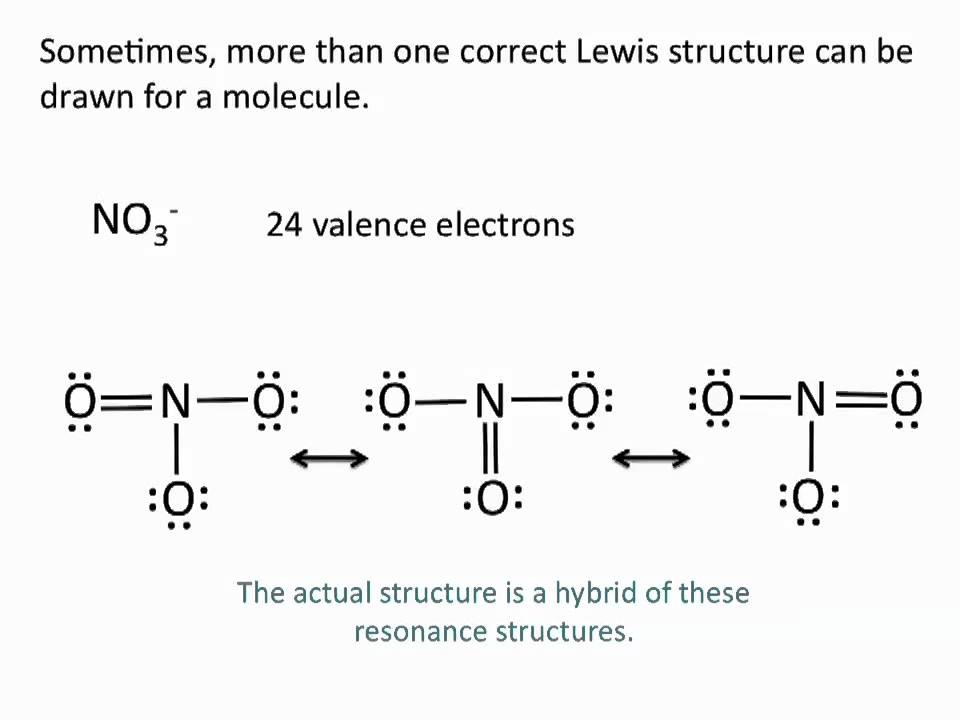
Drawing Lewis Structures Resonance Structures Chemistry Tutorial Youtube Chemistry Organic Chemistry Science Education
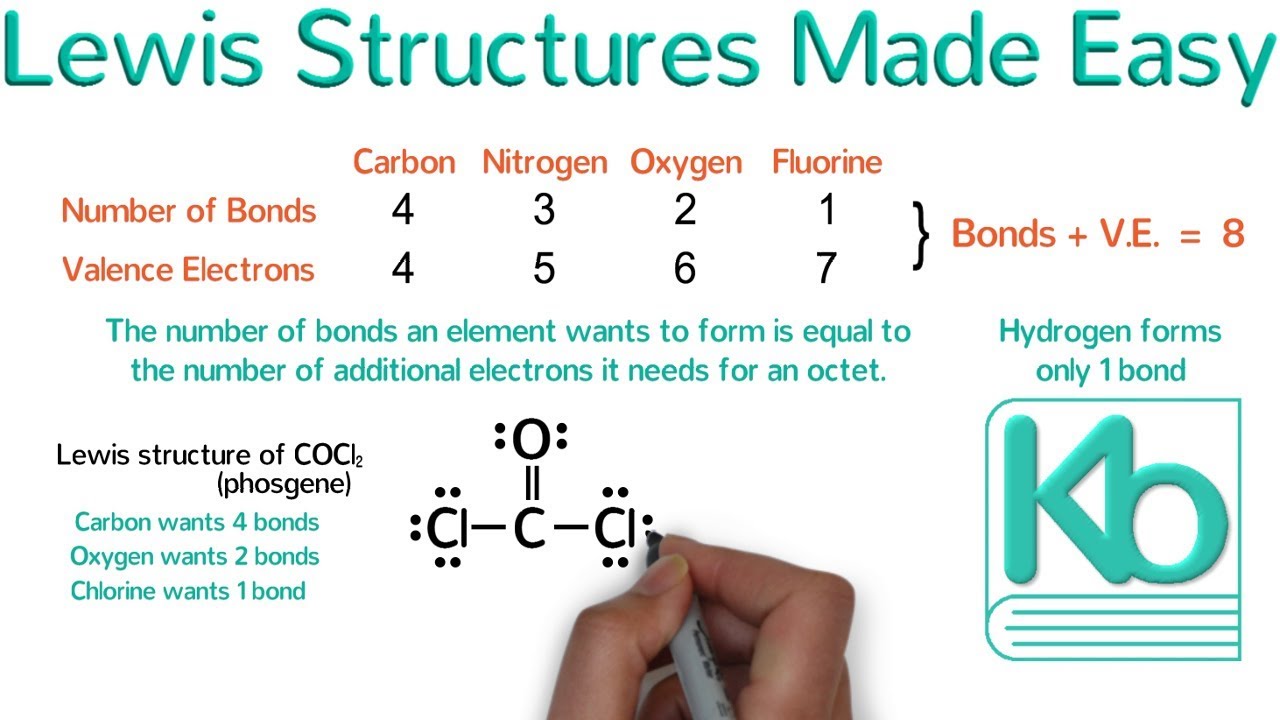
Lewis Structures Made Easy Examples And Tricks For Drawing Lewis Dot Diagrams Of Molecules Yo High School Chemistry Teaching Science Organic Chemistry Study

Calculating Molar Masses Teaching Chemistry Chemistry Worksheets Chemistry Lessons





