Brf3 Lewis Acid Or Base
See the answer See the answer See the answer done loading. Is bf3 A acid or base.

Autoionization Of Brf3 Non Aqueous Solvents In Inorganic Chemistry Acid And Base Chemistry Csirnet Youtube
The Brønsted-Lowry concept seeks to generalize Arhennius acidity in ways that allow all hydrogen ion transfers to be thought of as an acid-base.

Brf3 lewis acid or base. AlBr 3 Br Lewis b. In the following reactions BrF3 can act as a Lewis acid or as a Lewis base. Lewis acid strength of BF3 decrease due to 2p pi - 2p pi back bonding between the 2p orbitals of boron and flourine.
SO 2 ClO 3 Lewis f. Even though we use the term donate the electron pair does not leave the NH2 molecule it changes from a non. Part AIdentify the Lewis acids and bases in the reaction.
HClO 4 CH 3CN Lewis Brønsted-Lowry c. The solvent system acid base concept generalizes the Arhennius acid base concept by focusing on cation and anion generation in solution. Lewis acid accepts a pair of electron from lewis base in adduct eg lewis acids are diverse and boron trifluoride is a trigonal planar species which is an electron pair acceptor.
Hence there are three bonded pairs of electrons and two lone pairs in the Lewis structure of BrF3. Ni2 NH 3 Lewis d. Having a straw ie colorless to yellow appearance this chemical compound has several applications but also comes with a number of limitations and hazard issues.
Hence according to Lewis concept these are Lewis acids. What is the Lewis structure of BrF3. Lewis acid is a chemical species that reacts with a Lewis base to form a Lewis adduct.
Like the Brønsted-Lowry acid-base concept the solvent system acid base concept is way to generalize the Arhennius acid-base concept. ClF NH 3 Lewis e. A Lewis acid is a substance that accepts electrons whereas a Lewis base is a substance that donates electrons.
A Lewis acid can accept a pair of electrons from atom of same or different molecules known as Lewis base. In the following reactions BrF 3 can act as a Lewis acid or as a Lewis base. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
The Lewis Acid-base theory defines acids as species accepting pairs of electrons. B BrF3 Lewis base F- Lewis acid. BPt XeF 4 Lewis c.
BrF3 F- BrF4-a BrF3 Lewis acid F- Lewis base. The Lewis structure of BrF3 will have three bonds between Br-F represented by lines and four nonbonding electrons represented as four dots on the Bromine atom. C F- Lewis base BrF4- Lewis acid.
How many bonds can bromine form. B C2H4 ExplanationIn BF3 and FeCl3 molecules the central atoms have incomplete octet and in SiF4 the central atom has empty d-orbitals. 61 Acid Base Definition a.
Bromine will normally form one covalent bond. Boron Trifluoride BF3 can be found in a number of applications. Lewis acids are generally electron-poor with empty orbitals while Lewis bases are.
Part BThe salt BrF2AsF6 is soluble in BrF3. AgFs BrF3l à Ag BrF4- SbF5lBrF3làBrF2SbF6- This problem has been solved. Na is not a Lewis acid.
The correct option is. Normally lewis acids are species with vacant orbitalswhile lewis bases are species with lone pair of electron in the given reaction the BF3 has a vacant orbital draw the lewis structure of BF3 while F- has the lone pair of electron which it donates to the BF 3. HF C 3H7COOH Lewis Brønsted-Lowry 62 Acid Base Definition a.
The central boron atom in boron trichloride BCl3 is electron-deficient enabling the molecule to accept additional pairs of electrons and act as a Lewis Acid. Whats a Lewis Acid and Lewis Base. It represents a new class of acids.
Molecules where the central atom can have more than 8 valence shell electrons can be electron acceptors and thus are classified as. A Lewis acid can accept a pair of electrons from a Lewis base. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
XeO 3 OH Lewis This is not a Brønsted-Lowry reaction since the product connectivity is XeO 3OH. The boron in BF3 is electron poor and has an empty orbital so it can accept a pair of electrons making it a Lewis acid. Is C2H4 a Lewis acid or base.
An atom ion or molecule with an incomplete octet of electrons can act as an Lewis acid eg BF3 AlF3. BF3 is electron deficient molecule and has an empty p-orbital so it can accept a pair of electrons making it a Lewis acid. As a strong Lewis acid BF3s catalytic properties.
BrF3 Lewis Structure Molecular Geometry Hybridization and MO Diagram BrF3 known as Bromine Trifluoride is a fuming liquid consisting of inter-halogen combinations and bearing a pungent smell. Identify if it is the Lewis acid or base in the following two reactions. What is the role of bf3.
Is Na a Lewis acid or base. Is it an acid or base in this solvent.
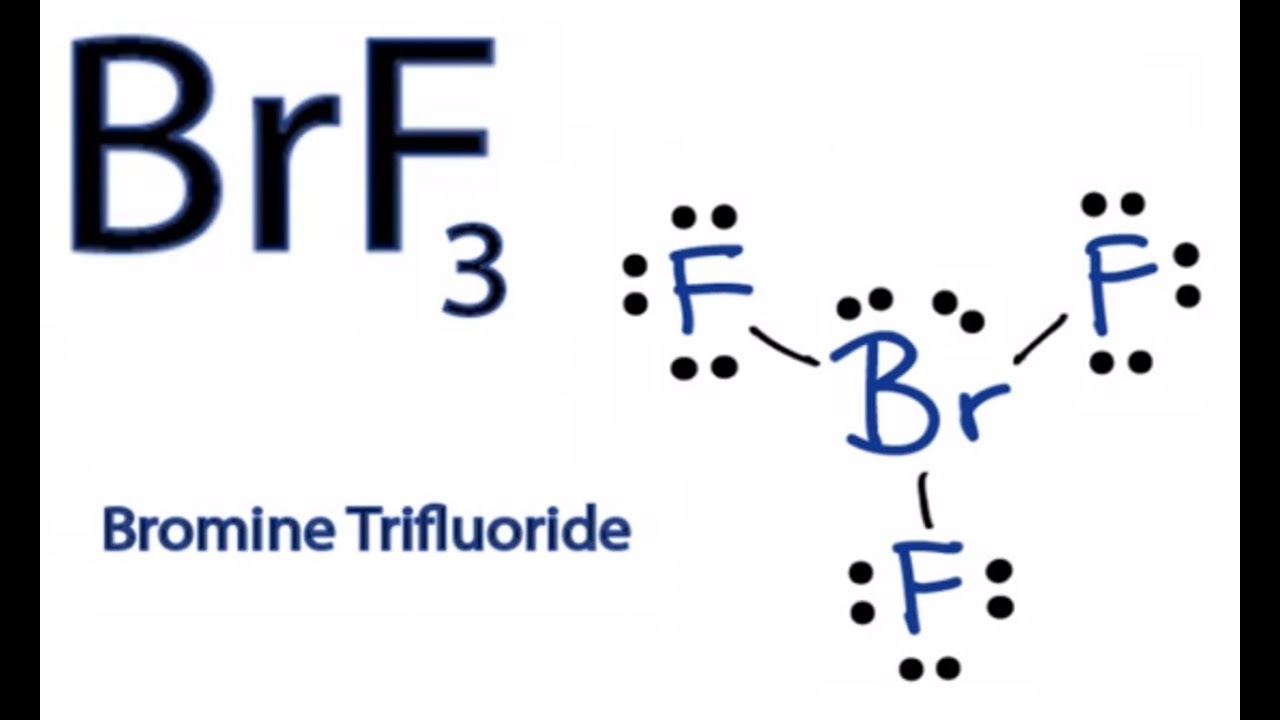
Lewis Structure For Brf3 Bromine Trifluoride
10 5 Bromine Trifluoride As A Solvent Chemistry Libretexts

Chemistry Of Brf3 Constructing The Cf2 Cf3 And Cf2h Groups Springerlink

Best Overview Is Brf3 Polar Or Nonpolar Science Education And Tutorials
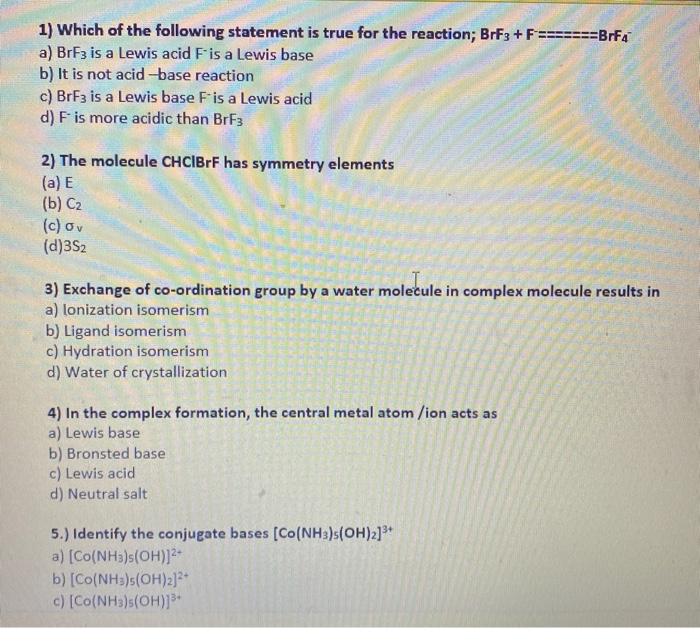
1 Which Of The Following Statement Is True For The Chegg Com
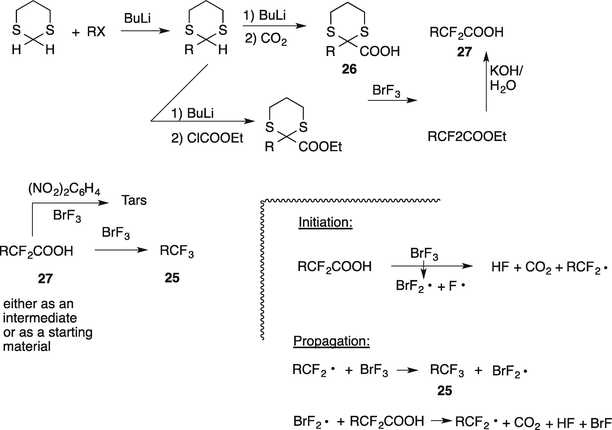
Chemistry Of Brf3 Constructing The Cf2 Cf3 And Cf2h Groups Springerlink
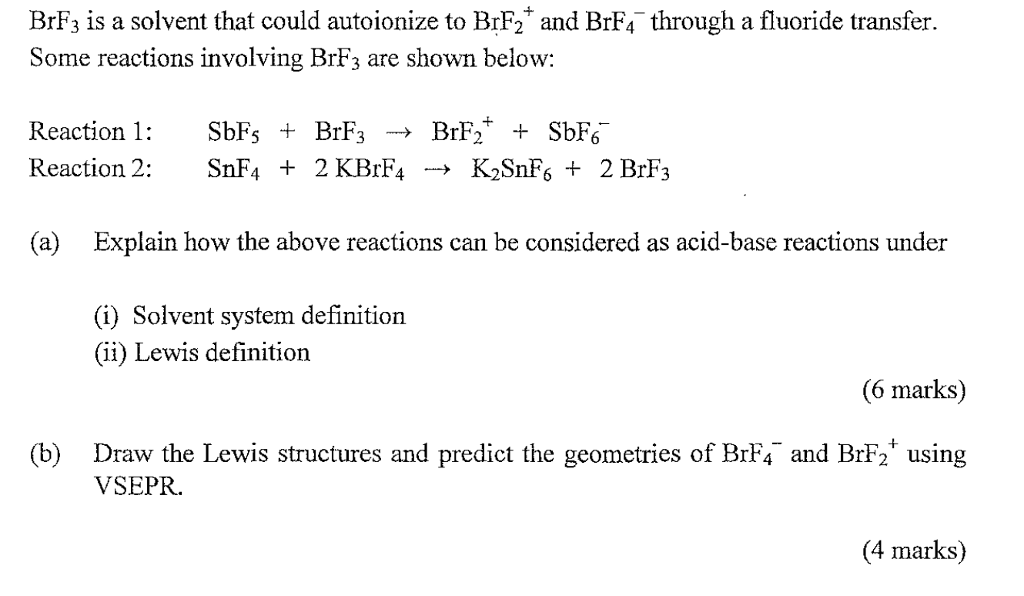
Brf3 Is A Solvent That Could Autoionize To Brf2 And Chegg Com
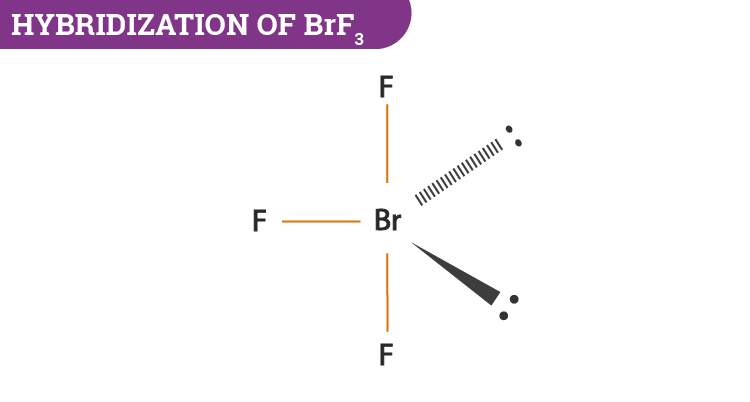
Hybridization Of Brf3 Hybridization Of Br In Bromine Trifluoride
Http People Brandeis Edu Ozerov Pages Genchem Lecture 20 2001 Pdf
Brf3 H2so4 Ch3oh Hf Please Sort The Given Chegg Com

Brf3 Polar Or Nonpolar Bromine Trifluoride Youtube

Brf3 Polar Or Nonpolar Bromine Trifluoride Youtube

Big Picture Perspective Acid Base Chemistry Is Highly Diverse Encompassing Not Only The Traditional H Oh Chemistry That Characterizes Aqueous Solutions Ppt Download

Acidbase And Donoracceptor Chemistry Chapter 6 Acids And

Best Overview Is Brf3 Polar Or Nonpolar Science Education And Tutorials
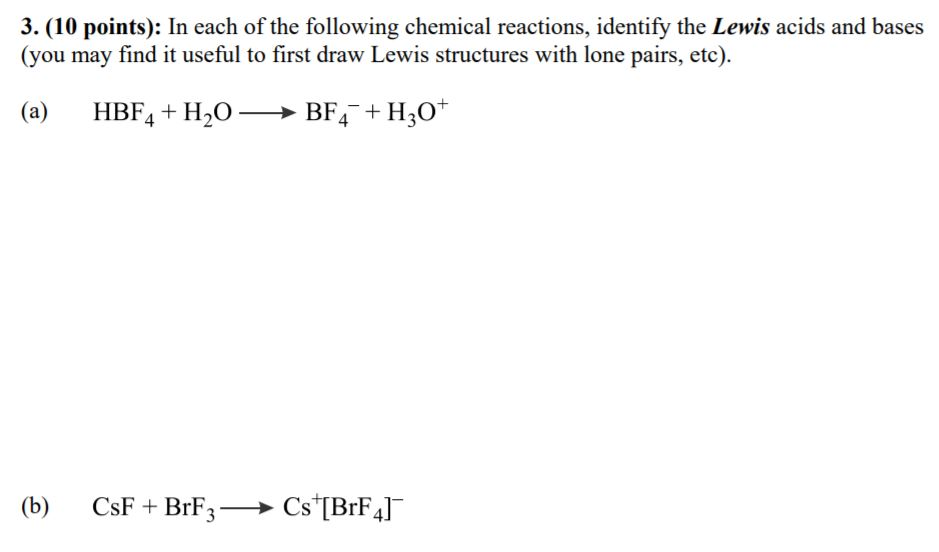
3 10 Points In Each Of The Following Chemical Chegg Com

Chemistry Of Brf3 Constructing The Cf2 Cf3 And Cf2h Groups Springerlink

Bromine Trifluoride As A Solvent Chemistry Of The Main Group Elements Openstax Cnx

Chemistry Of Brf3 Constructing The Cf2 Cf3 And Cf2h Groups Springerlink