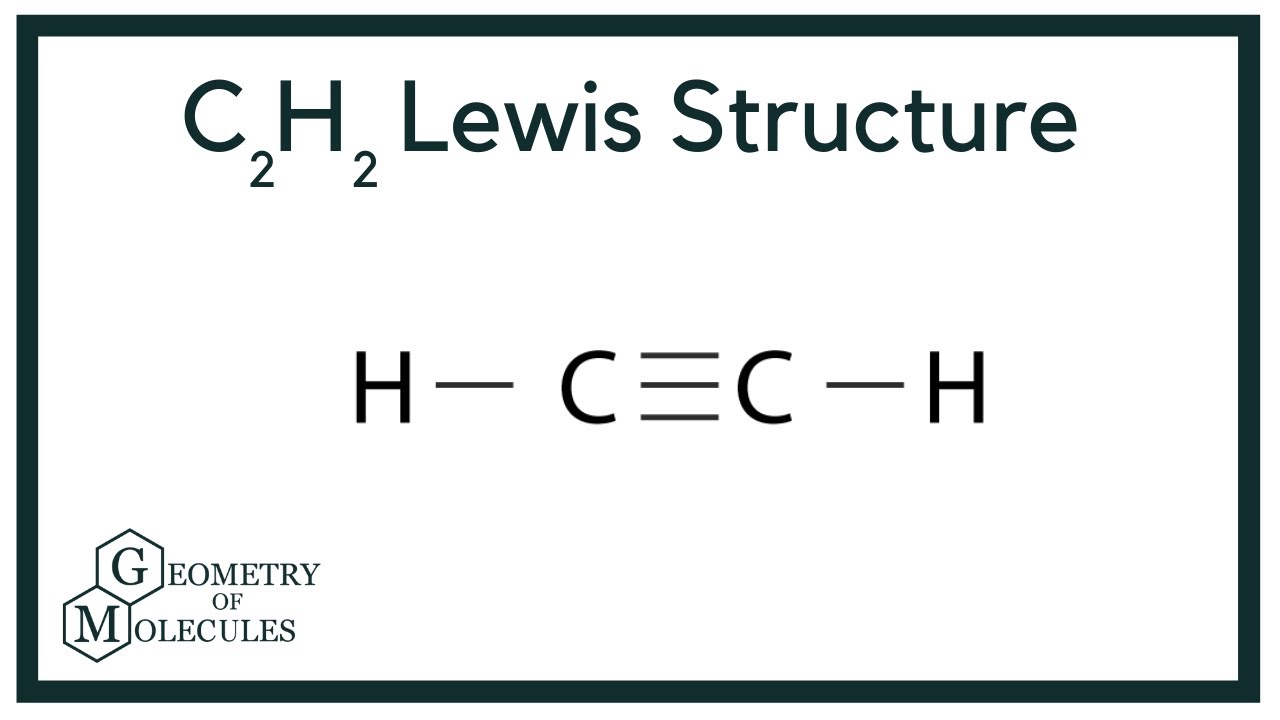
C2h2 Lewis Structure Bond Angle
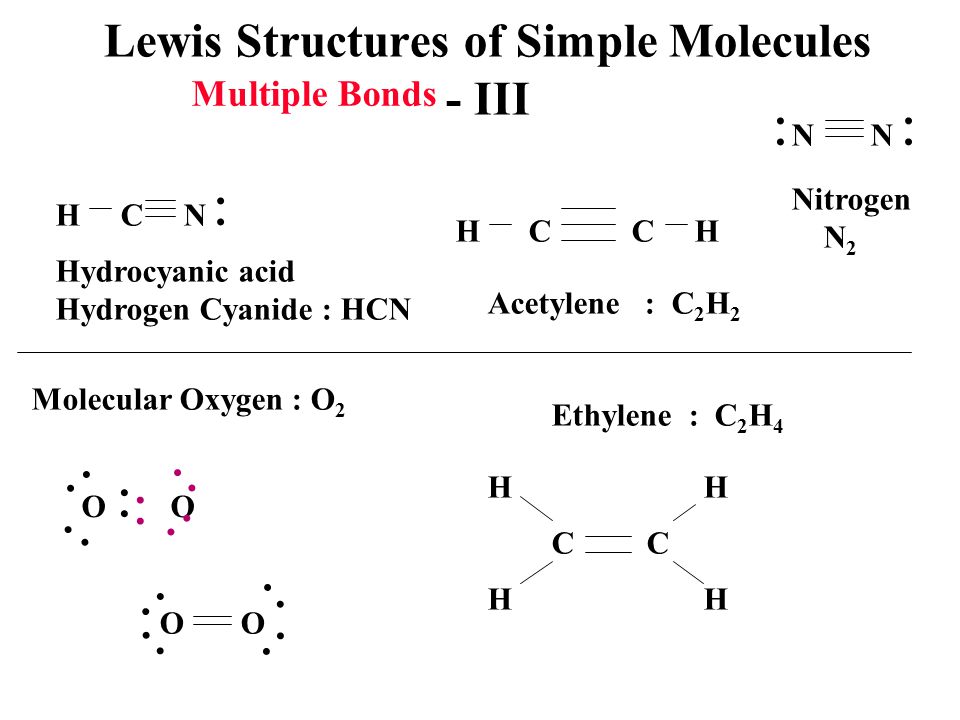
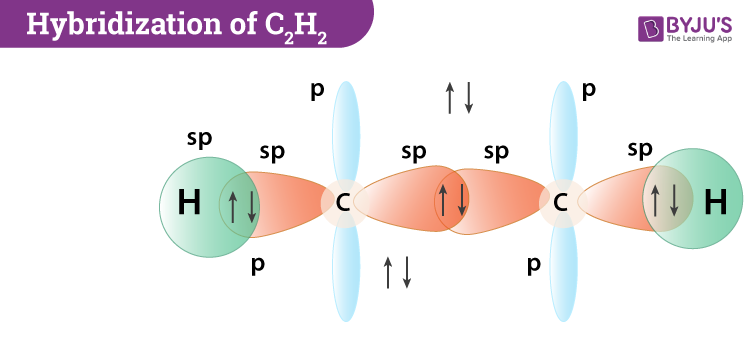
C2H4 Lewiss dot structure is very helpful to find Its molecular geometry because the lewis diagram helps us to determine how many bond pairs and lone pairs a molecule contains. N Central atom Number of Hybridi-zation Electron pair arrangement Molecular geometry around each central atom σ bond Lone pairs σ bond lone pairs π bonds C 2 2 2 sp Linear Linear Orbital box diagrams C.
C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle
Csonn t3 chemical bonding.
C2h2 lewis structure bond angle. The C-H bond distance is the longest in IIT 1989 a C2H2 b. It has 3 σ-bond and 2 π bond. When the ethane molecule is put together the arrangement around each carbon atom is again tetrahedral with approximately 1095 bond angles.
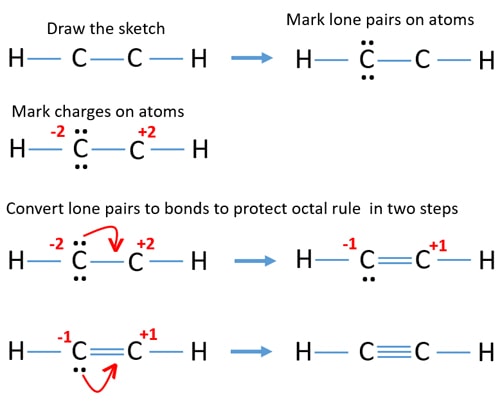
Total number of electron groups around each identical interior carbon atom. The molecules with a tetrahedral molecular geometry have bond angles of 1095 degrees which are typically affected by lone pairs of electrons. In drawing the Lewis structure for C2H2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds.
He 2 s 2 2 p 2 C. Is C2H2 trigonal planar. The solution is to share three pairs of valence electrons and form a triple bond between the Carbon atoms in C2H2.
He 2 s 2 p C. Since the double bond can be placed in more than one place without rearranging the atoms COCl2 exhibits resonance. Chemistry questions and answers.
Ethyne acetylene C2H2 HCCH Lewis structure using dots to represent bonding electrons. What is the hybridization and bond angle of a C2H4 molecule. Since there is only one possible lewis structure C2H2 does not have resonance.
The shape of the molecule is trigonal planar. Number of bonding groupspairs around an interior atom. A molecule has resonance if more than one lewis structure can be drawn for that molecule.
Bond angles are all 120. That is a tetrahedral arrangement with an angle of 1095. He sp 2p 180 Remember that double and triple.
You will then use the Lewis structure to draw a geometric sketch of the molecule including bond angles. C2H2 will have linear trigonal planar tetrahedral trigonal pyramidal bent trigonal bipyramidal seesaw T-shaped octahedral square pyramidal square planar electronic geometry and linear trigonal planar tetrahedral trigonal pyramidal bent trigonal bipyramidal seesaw T-shaped. Does COCl2 have resonance structures.
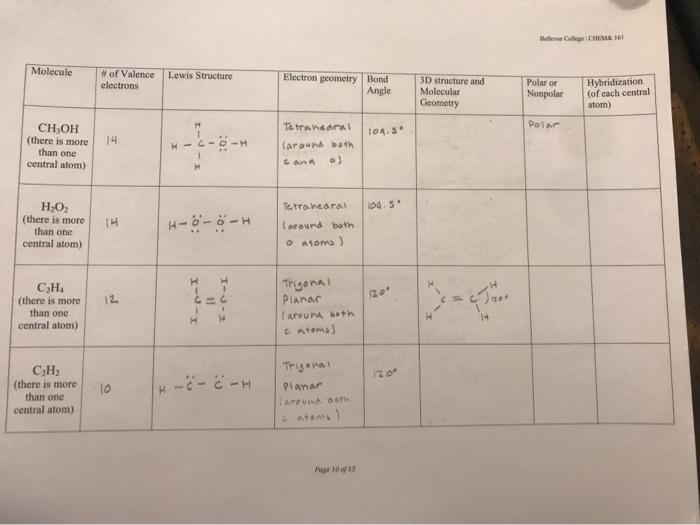
Does C2H2 have resonance. Number of lone pairs on an interior atom. For each of the following compounds draw a Lewis structure determine the bond angles and molecular shapes for all atoms.
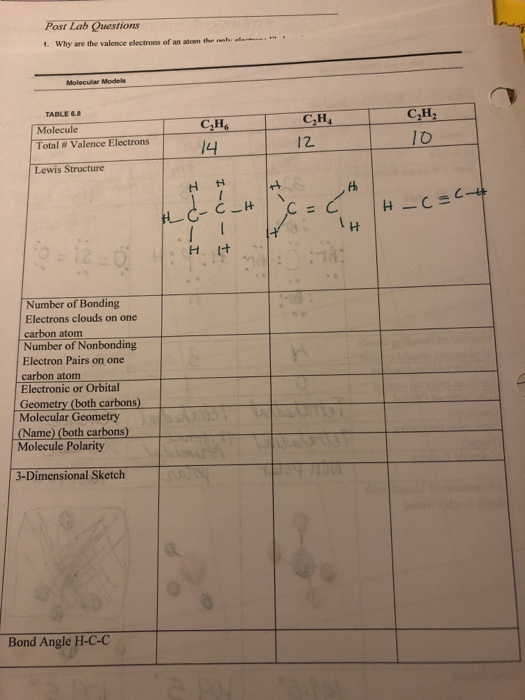
Why is C2H2 a triple bond. C2H6 Bond Angles. Lewis structure using lines to represent pairs of bonding electrons.
Each HCH bond angle is around 1175º because the presence of a double bond in between carbon atoms shrinks the angle between the HCH bond from 120º to 1175º. An electron group is either a single bond a double bond. C2h2 Lewis Structure C2H2 Lewis Dot Structure Geometry YouTube C2H2 Molecular Geometry Shape and Bond Angles see Hybridization Chapter 3.
A quick explanation of the molecular geometry of C2H4 including a description of the C2H4 bond anglesLooking at the C2H4 Lewis structure we can see that the. C 2 H 2 Lewis structure HC CH VSEPR sketch with bond angles HC CH Polar YN. You will predict the molecular geometry of each molecule by first counting the number of electron groups around the central atom.
C2h2 Lewis Formula lewis dot diagram structure electrons valence drawing cis dichloroethene lewis structure dot bond between double determine geometry molecular hybridization bond ethyne acetylene angle angles linear triple orbitals carbon lewis structure draw. For each of the following compounds draw a Lewis structure determine the bond angles and molecular shapes for all atoms. Lone pair of electrons can change the bond angles due to their repulsive forces but here in C2H6 as there are no lone pairs in the molecule the bond angles in C2H6 is 1095 degrees.

Molecular Geometry Of Acetylene Chemistry Stack Exchange
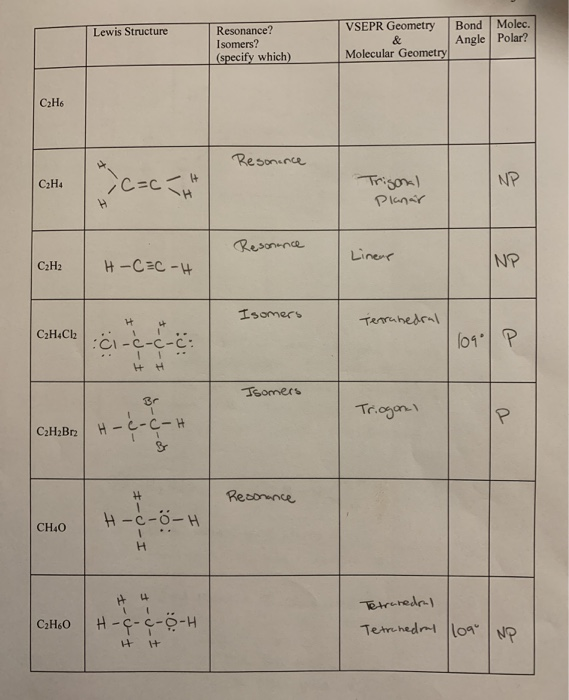
Lewis Structure Bond Molec Angle Polar Resonance Chegg Com
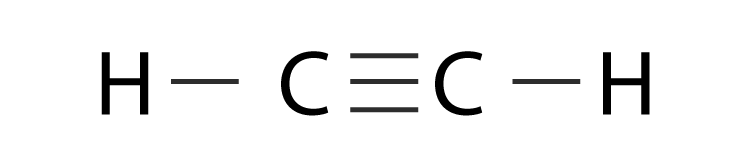
Hybridization Of C2h2 Hybridization Of C In Acetylene Ethyne

C2h2 Molecular Geometry Shape And Bond Angles See Description For Note Youtube
C2h2 Acetylene Ethyne Lewis Structure

C2h2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist

C2h2 Lewis Dot Structure Geometry Youtube

C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle
Hybridization Of C2h2 Hybridization Of C In Acetylene Ethyne
C2h2 Molecular Geometry Shape And Bond Angles See Description For Note دیدئو Dideo

Is C2h2 Polar Or Nonpolar All About C2h2 Polarity

C2h2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist
Report Sheets For Polar Bonds Draw In The Dipoles Chegg Com

Chapter 10 The Shapes Of Molecules Ppt Video Online Download

Which Is The Correct Lewis Structure For Acetylene C2h2 Brainly Com
C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle
Post Lab Questions 1 Why Are The Valence Electrons Chegg Com
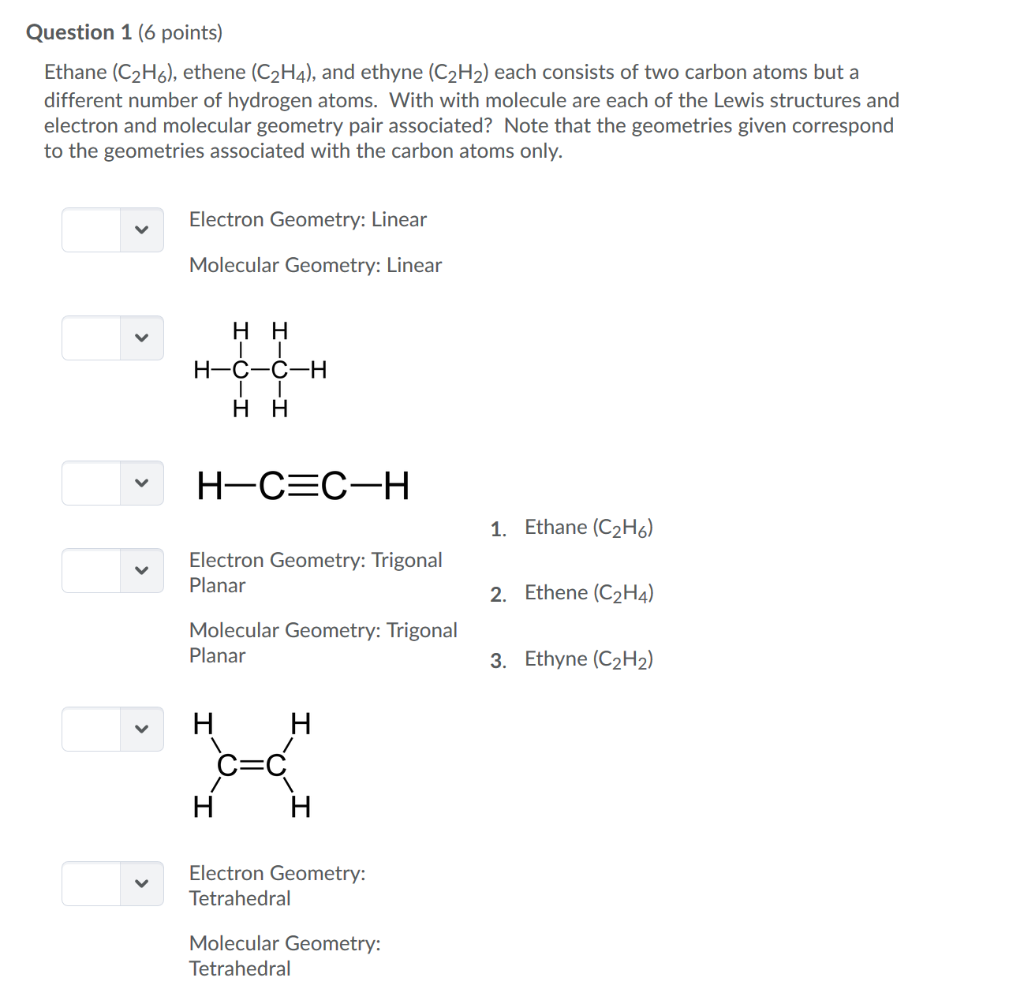
Question 1 6 Points Ethane C2h6 Ethene C2h4 Chegg Com

Hcch Lewis Structure How To Draw The Lewis Structure For The Hcch Youtube