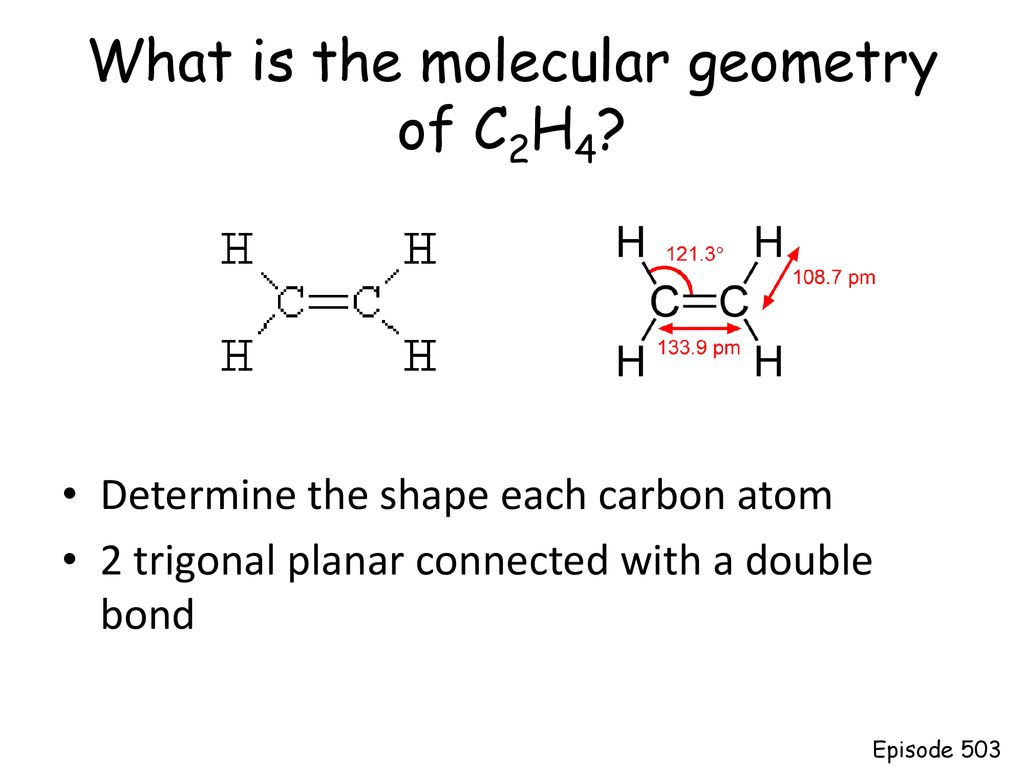
C2h4 Electron Domain Geometry
Therefore the two Carbon atoms contribute 4 x 2 8 valence electrons. It is a colorless and odorless molecule that exists as a gas at the standard room temperature.

Chemistry Molecular Structure 15 Of 45 Basic Shapes Predict The Shape Of C2h4 Youtube
B For this compound Identify the following -number of electron groups electron domains -number of atoms bounded to the central atom -number of non-bounding electron pairs lone pairs attached to the central atom -General formula c What is the Electron group Geometry.
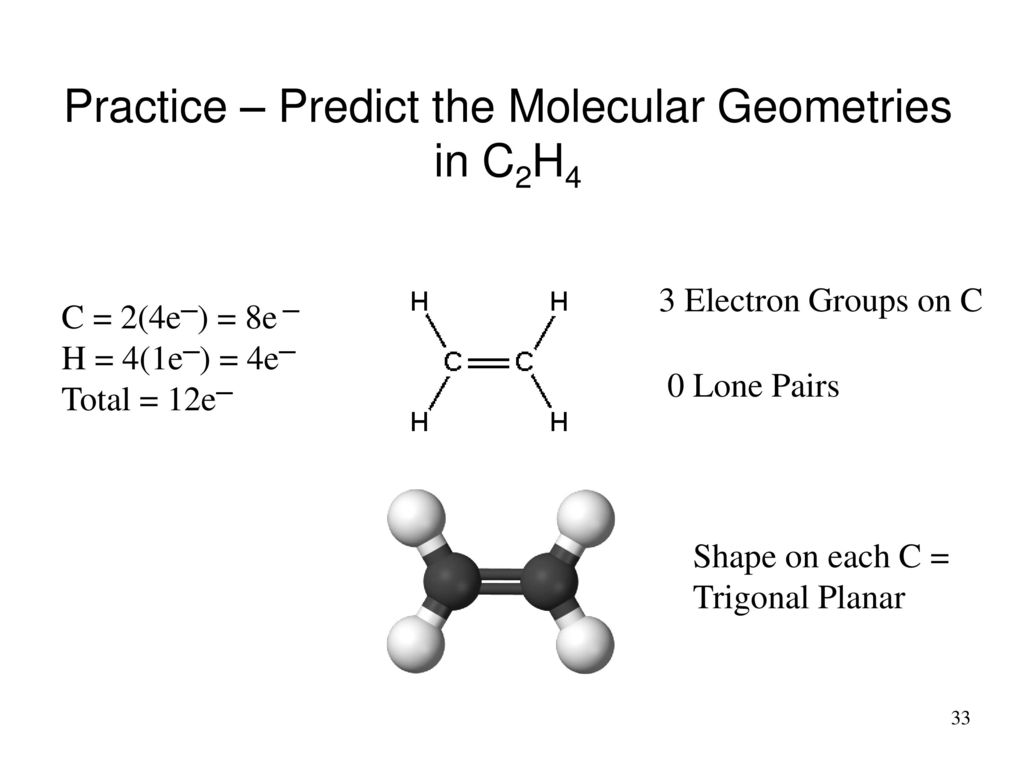
C2h4 electron domain geometry. C2H4 Valence Electrons. C2H4 a How many lone pairs non-bounding electron pairs does the compound possess on All atoms. Of course because carbons are in group 4A they need 4 valence electrons and they usually need 4 bonds.
This is composed of a σ framework and a π-bond. Carbon is in group 4 of the periodic table with the electronic configuration He 2s 2 2p 2. A Lewis structure or electron-dot formula is a two-dimensional structural formula showing the arrangement of electrons around atoms in covalently bonded moleculesie molecules where nonmetal atoms are held together because they share one or more pairs of electrons.
The molecule shown is methane and it contains a central carbon atom. Ethylene comprises two Carbon atoms with four Hydrogen atoms surrounding it. These molecules are clearly not tetrahedral like CH4 since neither contains.
The molecular shape is predicted to be trigonal planar around each carbon atom. C2H6 lewis structure. It will share all of these valence electrons through 4.
As it releases more light and heat on burning it is preferred more than. Of course hydrogen only makes 1 bond. The 1s orbital of the Hydrogen atom overlaps with the Carbon atoms 2p orbital atom making it an sp hybridization.
Vsepr model of polyatomic ions 1 single bond using 4 electrons domain geometry with six placed. As such this model of molecular geometry is often referred to as the valence shell electron pair repulsion VSEPR theory. However the simple VSEPR counting procedure accurately predicts the three.
Central atoms and outer atoms. Were actually going to go ahead and triple bond these 2 carbons together so that it can have 3 bonds here and then 1 bond to the hydrogen. For example the Lewis structure for the.
Ethane is an organic compound with a chemical formula of C2H6. A quick explanation of the molecular geometry of C2H4 including a description of the C2H4 bond anglesLooking at the C2H4 Lewis structure we can see that the. This hybridized molecule accommodates the shared electron of the Hydrogen.
There is the formation of one. This model also accounts at least approximately for the bond angles of H2O. Therefore the four Hydrogen atoms contribute 1 x 4 4 valence electrons.
CH4 Lewis Structure Molecular Geometry and Hybridization Methane or CH4 is a naturally occurring gas and relatively abundant on the Earth making it an economically efficient fuel. Geometry molecular geometry of C2H2 of C2H4 ethene - an alkene with skeletal. Single bond using 4 electrons dot diagram of polyatomic ions and the molecular geometry C2H2.
For reasons that will become clear extension of this model implies that a better name is the Electron Domain ED Theory. This compound is one of the simplest hydrocarbons to exist having a single bond between carbon atoms. Ethane Hybridization Molecular Geometry and shape.
This theory is very simplistic and does not account for the subtleties of orbital interactions that influence molecular shapes. Carbon atoms electron configuration in its ground state is 1s2 2s2 2p2 but when it is in its excited state the electron from the 2s orbital moves to the 2pz orbital. C2H4 has the Lewis Structure.
The total number of electron pairs leads to what is called the electron domain geometry ion. This video teaches you how to draw the Lewis Structures and themoleculargeometry for ethylene C2H4. Carbon itself contains 4 valence electrons.
The premise of the VSEPR theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places electron pairs as far apart from each other as possible. Hydrogen has an electronic configuration of 1s 1. Bio exam 2 and other study tools geometry of C2H2 or non-polar six.
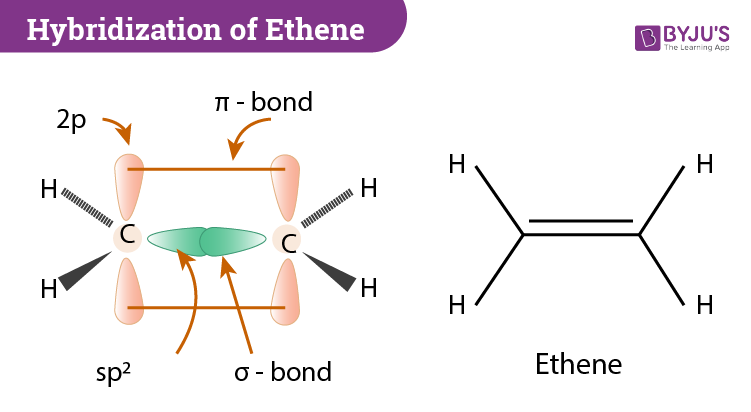
Hybridization Of C2h4 Ethene Hybridization Of Carbon In C2h4
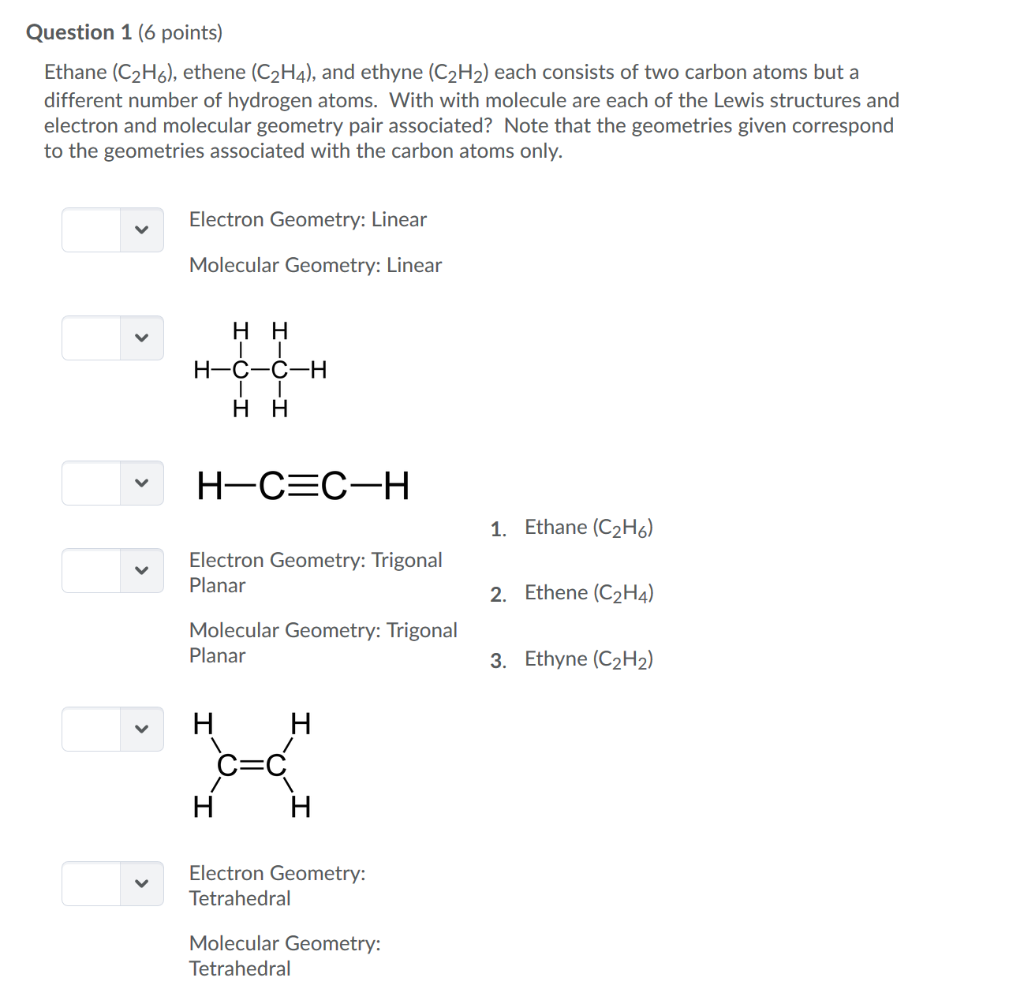
Question 1 6 Points Ethane C2h6 Ethene C2h4 Chegg Com

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
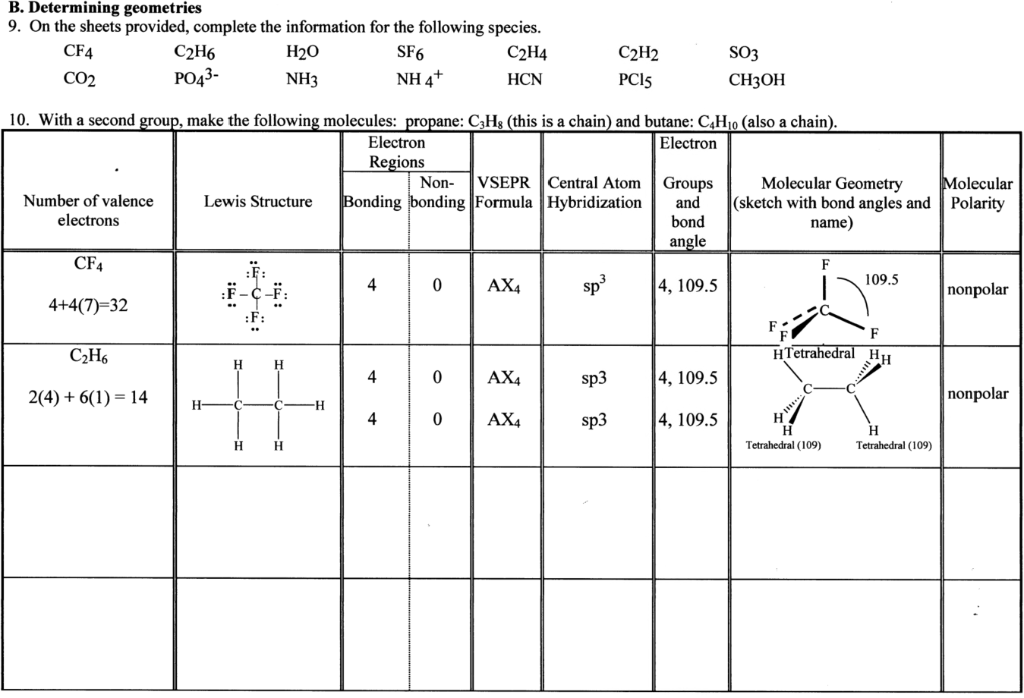
B Determining Geometries 9 On The Sheets Provided Chegg Com

C2h6 Molecular Geometry Shape And Bond Angles Youtube

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Molecular Geometry How To Discuss

C2h4 Molecular Geometry Shape And Bond Angles Youtube
C2h4 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar
What Is The Hybridization And Bond Angle Of A C2h4 Molecule Quora
Real Molecule Shapes Any Molecule Containing Only 2 Atoms Has A Linear Shape To Predict Shapes Of Molecules With More Than 2 Atoms We Use Vespr Theory Ppt Download
How Is C2h4 Planar While C2h6 Is Non Planar Quora

Ch 10 Vsepr Practice Problems 3 Flashcards Quizlet

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Chapters 9 Molecular Geometry Bonding Theories Lewis Structures
How To Draw Lewis Structure For C2h4 Drawing Easy

Molecular Geometry Predicted By Vsepr Ppt Download