Draw The Lewis Structure For Sef4
Lets do the Lewis structure for SeF4 Lewis Structure. The steric number of selenium central atom in the SeF4 molecule is 5 thus it forms Sp 3 d hybridization.
Http Www Msubillings Edu Sciencefaculty Handouts Wiles Chem 20116 Solution 20set 202 Pdf
Youll have a pair of electrons left over after filling octets of the F atoms.

Draw the lewis structure for sef4. The electron arrangement for five electron pairs is trigonal bipyramidal. 5 The VSEPR notation is AX₄E. As one can probably see there is one sulfur atom in this compound and four fluorine atoms.
Since there are seven Fluorine F atoms it will be necessary. Youll have a pair of electrons left over after filling octets of the F atoms. A step-by-step explanation of how to draw the SeF2 Lewis Dot StructureFor the SeF2 structure use the periodic table to find the total number of valence elec.
Draw the Lewis structures for the following molecules or ions for 1 SF6 2 SeF4. To know the total valence electrons of this compound we need to know the valence electrons of both the atoms individually. The way to determine the molecular geometry of SeF4 is to first draw the Lewis Dot Structure.
Se on the periodic table is in Group Six so it has six valence electrons. Quiz your students on lewis dot diagram structure for sef4 molecular geometry hybridization polar or nonpolar using our fun classroom quiz game quizalize and personalize your. The structures in which a chemical bond is drawn between the central atom and the side atoms and the free electrons are also expressed as dots around the atoms are called the.
As with SF₄ the shape is a see-saw. The total valence electron is available for drawing the Selenium tetrafluoride SeF4 lewis structure is 34. Fluorine seven valence electrons but we have four of those.
What is the shape of SeF4. In the SeF 4 is Lewis structure Selenium Se is the least electronegative atom and goes in the center of the structure. What is the geometry of SeF4.
A step-by-step explanation of how to draw the SF4 Lewis Dot Structure Sulfur tetrafluorideFor the SF4 structure use the periodic table to find the total n. Draw Lewis Structures For SeF_4 And SeF_6 And Include All Electron Lone Pairs. 4 The hybridization that corresponds to five electron pairs is sp³d.
The molecular shape considers only the Se-Br bonds. A step-by-step explanation of how to draw the SbF4 - Lewis Dot StructureFor the SbF4 - structure use the periodic table to find the total number of valence. SeF4 is a polar molecule because of asymmetrical geometry that causes the non-uniform distribution of charge in the molecule.
Very toxic by inhalation. What are the approximate bond angles made by the atoms in this structure. Lewis Dot Structure For Sef4.
Similarly you may ask what is the Lewis structure for SeF4. 6 The bond angles are. So lets multiply that out.
Drawing the Lewis Structure for SeF 4. And to draw the Lewis structure of SF4 we first need to know the total number of valence electrons in this molecule. The Lewis structure is like that of SeF₄.
SeF4 is Lewis structure with Selenium which can hold more than 8 valence electrons. Note that Se can hold more than eight valence electronsFor the SeF6 Lewis structure. As Xenon has two lone pairs of electrons it will take up a structure that helps these lone pairs avoid the repulsion forces.
3 The bulky lone pair occupies an equatorial position. A step-by-step explanation of how to draw the SiH4 Lewis Dot Structure Silicon TetrafluorideFor the SiH4 structure use the periodic table to find the tota. A step-by-step explanation of how to draw the SeF4 Lewis Dot Structure Selenium TetrafluorideFor the SeF4 structure use the periodic table to find the tot.
Six plus twenty eight equals thirty-four total valence electrons. Is The Octet. Draw the Lewis structure for SeF4 and answer the following questionsHow many valence electrons are present in this compoundHow many bonding electrons are present in this compoundHow many lone pair non-bonding electrons are present in this compoundIs SeF4 a polar or non-polar compound.
SeF 4 is Lewis structure with Selenium which can hold more than 8 valence electrons. A step-by-step explanation of how to draw the CF4 Lewis Dot Structure Carbon TetrafluorideFor the CF4 structure use the periodic table to find the total n. A step-by-step explanation of how to draw the SeF6 Lewis Structure.

Sef4 Lewis Structure How To Draw The Lewis Structure For Sef4 Youtube

Consider The Molecule Sef4 A Draw The Lewis Structure B What Is The Hybridization Of Se C What Is The Electron Geometry D What Is The Molecular Geometry E What Degree Angles
Is Sef4 Polar Or Nonpolar Quora
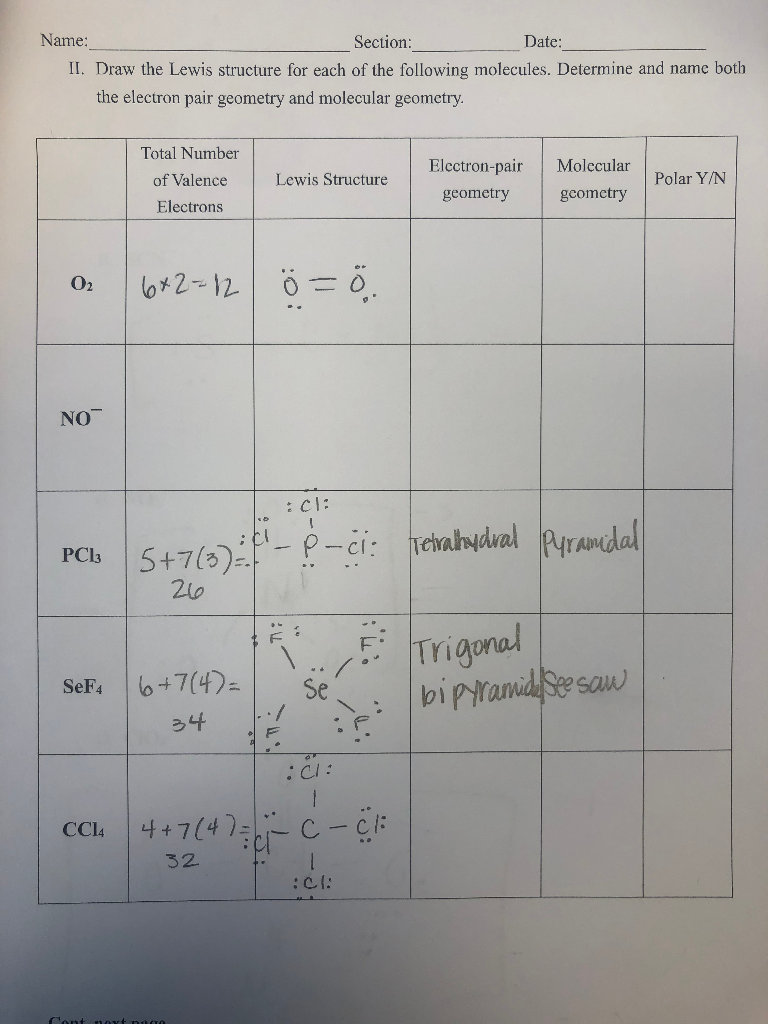
Draw The Lewis Structure For Each Of The Following Chegg Com

Write Lewis Structures For Sef4 And Sef6 Is The Octet Rule Satisfied For Se Study Com

Hcch Lewis Structure How To Draw The Lewis Structure For The Hcch Youtube
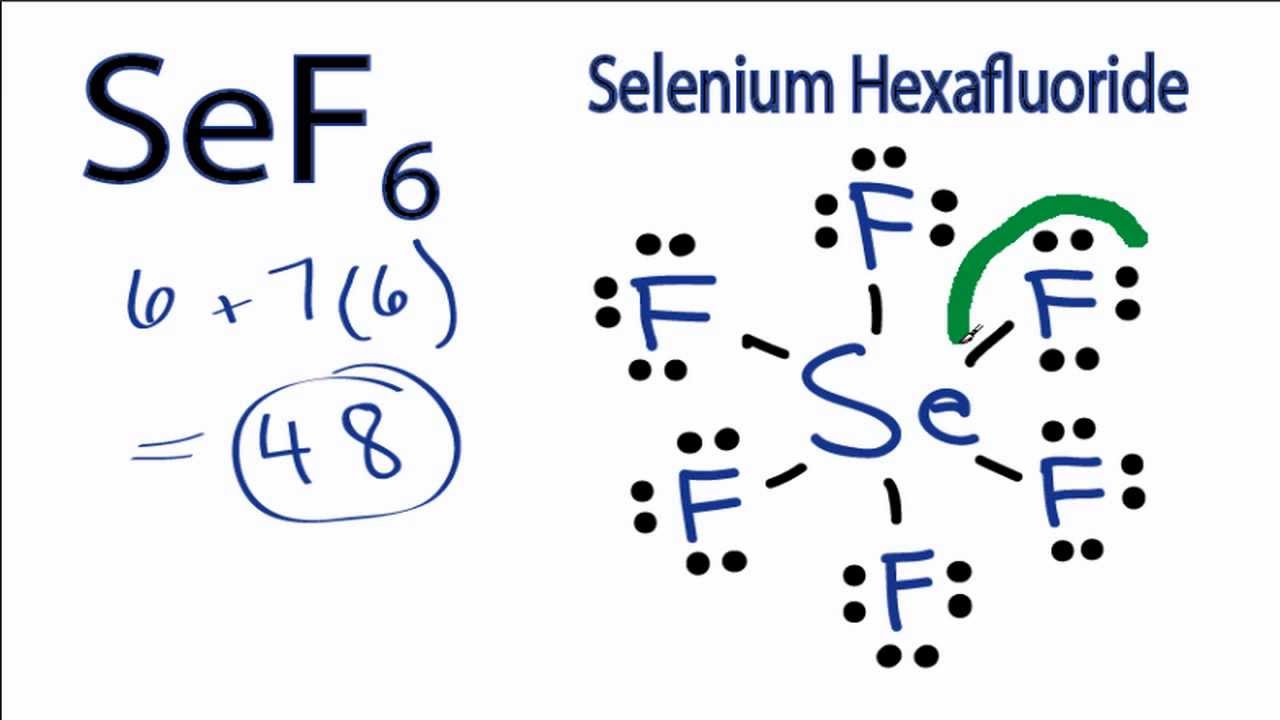
Sef6 Lewis Structure How To Draw The Lewis Structure For Selenium Hexafluoride Youtube

Draw The Lewis Structure Of Selenium Tetralfluoride Sef4 Clutch Prep
Lewis Structures And Shapes Of Molecules Teaching Resources

Sef4 Lewis Structure How To Draw The Lewis Structure For Sef4 Youtube

What Is The Molecular Geometry Of Sef4 How Is It Determined Quora

Sf4 Lewis Structure How To Draw The Lewis Structure For Sf4 Youtube

Determine The Molecular Geometry Of Sef4 Clutch Prep

Sef4 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle
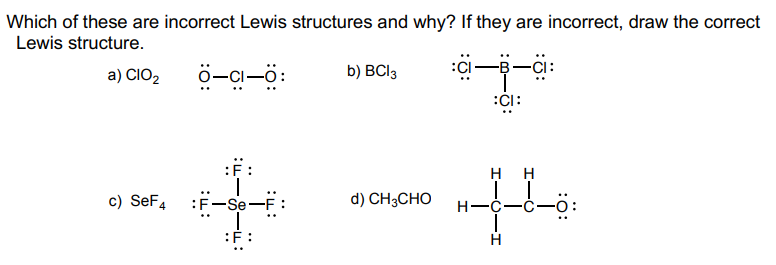
Which Of These Are Incorrect Lewis Structures And Chegg Com
Sf4 Molecular Geometry Lewis Structure Bond Angles And Polarity

How To Calculate The Formal Charges For Sf4 Sulfur Tetrafluoride Youtube

Krf4 Lewis Structure How To Draw The Lewis Structure For Krf4 Krypton Tetrafluoride Youtube
What Is The Molecular Geometry Of Sef4 How Is It Determined Quora
