Ethylene Molecular Geometry Name
It is also called Ethene or Polyethylene or Etileno. Ethylene is a hydrocarbon which has the formula C 2H 4 or H2CCH2.

Structure And Bonding In Ethene The Pi Bond Chemistry Libretexts
Tetrahedral 2 trigonomial pyramidal 3linear 4.
Ethylene molecular geometry name. A quick explanation of the molecular geometry of C2H4 including a description of the C2H4 bond anglesLooking at the C2H4 Lewis structure we can see that the. The 𝝅CC stands for LUMO Lowest Unoccupied Molecular Orbital. 1213 Molecular Geometry of C 2 H 4.
Polar because it is not symmetrical. Naphthalene is an organic compound with formula C 10H 8. 1 Answer to What is the shape around each atom in ethylene H2C CH2.
As an aromatic hydrocarbon naphthalenes structure consists of a fused pair of benzene rings. The 𝝅CC stands for Highest Occupied Molecular Orbital or HOMO. The correct Lewis structure for ethene is shown below.
Name of the molecule. A formaldehyde molecule is trigonal planar because it has an atom at the end of each electron cloud. Ethene is the formal IUPAC name for H2CCH2 but it also goes by a common name.
In sulfur dioxide there are three electron clouds around the sulfur. Hybridization of central atom. On the first hand it minimizes repulsion between electrons due to electrostatic.
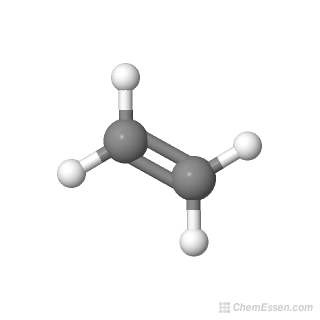
Ethylene is an unsaturated organic compound with the chemical formula C2H4. Only two of these connect two atoms. It is the simplest alkene.
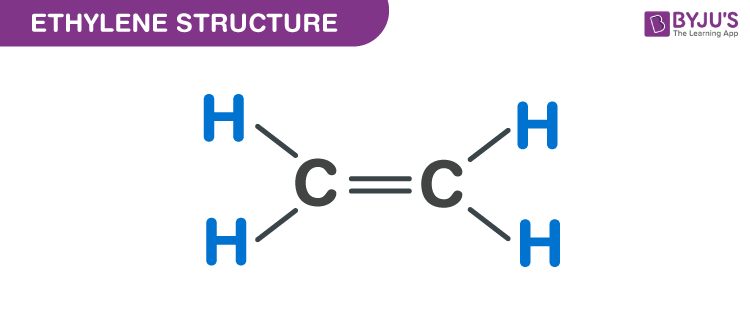
Pronounced vesper model provides some useful tools for predicting molecular geometries. Equal sign represent double bond 1. Ethylene C 2 H 4 No.
The simple view of the bonding in ethene. Each line in this diagram represents one pair of shared electrons. Ethene is actually much more interesting than this.
These p-orbitals will undergo parallel overlap and form one σ σ bond with bean-shaped. The valence shell electron pair repulsion VSPER. Non polar because it is symmetrical.
This model proposes that electrons are arranged around atoms in pairs such that they are kept as far away as possible. It has one double bond and is the simplest member of the alkene class of hydrocarbons. The VSPER model is based on two important principles.
In the molecule the oxygen-sulfur-oxygen atoms make a 120 angle. It is best known as the main ingredient of traditional mothballs. The above diagram shows the Molecular OrbitalMO diagram of etheneethylene.
Much of this production goes toward polyethylene a widely used plastic containing polymer chains of ethylene units in various chain lengths. Of valence electrons 2 x 4 4 x 1 12 valence electrons. In the molecule ethene both carbon atoms will be sp2 hybridized and have one unpaired electron in a non-hybridized p orbital.
Tetrahedral 2 trigonomial pyramidal 3linear 4. These are all single bonds but the bond in molecule C is shorter and stronger than the one in B which is in turn shorter. Ethylene is widely used in the chemical industry and its worldwide production exceeds that of any other organic compound.
Ethene or ethylene H 2 CCH 2 is the simplest alkene example. At a simple level you will have drawn ethene showing two bonds between the carbon atoms. Ethene is a hydrocarbon which has the formula C 2 H 4 or H 2 CCH 2It is a colorless flammable gas with a faint sweet and musky odour when pure.
The antibonding orbital remains empty. It is a colorless flammable gas with a faint sweet and musky odor when pure. It is the simplest polycyclic aromatic hydrocarbon and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 008 ppm by mass.
Chime in new window Since a double bond is present and each carbon is attached to 3 atoms 2 H and 1 C the geometry is trigonal planar. The whole molecule is planar and its shape resembles two triangles joined point to point. Trigonal planar equal sign represent double bond 1.
The ethylene molecule has trigonal planar geometry around each of its carbon atoms. C 2 H 4 is a simplest alkene with chemical name Ethylene. Ethanol CH3CH2OH or C2H6O CID 702 structure the structural formulas the organization of the individual molecules in space are different between ethanol.
Difference Between Ethane And Ethene Definition Properties Applications Similarities

Hybridization Structure Of Ethane Mcc Organic Chemistry

C2h4 Molecular Geometry Shape And Bond Angles Youtube

A Powerful New Tool For Assembling Biomolecules Research Companies Marketing Marketing Trends

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Is C2h4 Polar Or Nonpolar All About C2h4 Polarity
Difference Between Ethene And Ethyne Definition Properties Reactions Applications

Illustrated Glossary Of Organic Chemistry Ethylene Glycol
Ethylene Structure C2h4 Over 100 Million Chemical Compounds Mol Instincts
Ethylene C2h4 Structure Molecular Mass Physical Chemical Properties Uses
C2h4 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar



