How To Find Nonbonding Electron Pairs
Yes there are ways one could claim to calculate an angle between two non-bonding electron pairs. Whereas lone pairs are the pairs of electron on an atom that do not participate in the bonding of two atoms.

1 Determine The Number Of Bonding Electrons And The Number Of Nonbonding Electrons In The Structure Of Co 2 2 Draw The Main Lewis Structure Of Nof 3 Determine The Number Of Bonding
Example 1 Phosphate ion PO4 -3 charge.

How to find nonbonding electron pairs. Lisbon Mediterranean Sea The Alps Rome Athens 1. The total number of valence electrons in an atom is equal to the sum of the electrons that form bonds bonding electrons and the nonbonding electrons. The oxygen atom will therefore be tetrahedrally coordinated meaning that it sits at the center of the tetrahedron.
Try to satisfy the octets of the atoms by distributing the remaining valence electrons as nonbonding electrons. Electrons in molecular non-bonding orbitals can undergo electron transitions such as nσ or nπ transitions. How many non-bonding electron pairs are there at the P atom of the Lewis structure of PCIS.
How many bonding and non-bonding electron pairs are found in the BF3 molecule. Questions in other subjects. How many non-bonding electron pairs are there at the 5 atom of the Lewis structure of SF6.
The following initial rates of reaction have been measured for. Molecular orbitals come from the linear combination of atomic orbitals. A The X in AX4E A X 4 E represents bonding pairs.
Write the skeleton structure of the molecule. The S has 1 nonbonding pair 1 O has 1 nonbonding pair the other O has 2 nonbonding pairs. A nonbonding electron is an electron in an atom that does not participate in bonding with other atoms.
For example nπ transitions can be seen in ultraviolet-visible spectroscopy of compounds with carbonyl groups although absorbance is fairly weak. Ammonium ion NH4 reacts with nitrite ion NO2- to yield nitrogen gas and liquid water. The term can refer to either a lone pair in which the electron is localized and associated with one atom or to a non-bonding orbital in which the electron.
How many nonbonding electron pairs are in a hypochlorite ion. Determine the total number of valence electrons. A non-bonding orbital NBMO is a molecular orbital for which the addition or removal of an electron does not change the energy of the molecule.
See the answer See the answer See the answer done loading. To identify lone pairs in a molecule figure out the number of valence electrons of the atom and subtract the number of electrons that have participated in the bonding. с C en.
They are also known as lone pairs or unshared pair of electrons. The s orbital of H can overlap with the 2p_z orbital of fluorine to form a bonding σ and an antibonding. Therefore there are 4 bonding pairs in the molecule.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. In the Lewis structure of a molecule the nonbonding electrons are those that are not utilized in the formation of chemical bonds. In a simple diatomic molecule such as HF F has more electrons than H.
Use two valence electrons to form each bond in the skeleton structure. How many nonbonding electron pairs lone pairs on AS. This problem has been solved.
Asked Sep 12 2016 in Chemistry by Helena. B The E in AX4E A X 4 E represents nonbonding pairs. In the water molecule AX 2 E 2 the central atom is O and the Lewis electron dot formula predicts that there will be two pairs of nonbonding electrons.
The shape will be Y-shaped. Nonbonding electrons are those valence electrons in an atom that are not involved in bond formation. A 1 bonding and 3 non-bonding B 2 bonding and 2.
As Mithoron points out this ChemSE question illustrates how quantum chemical calculations and photoelectron spectroscopy both demonstrate the non-equivalence of the lone pairs of ceH2O an analysis which presumably applies equally well to the analogous ceH2S.
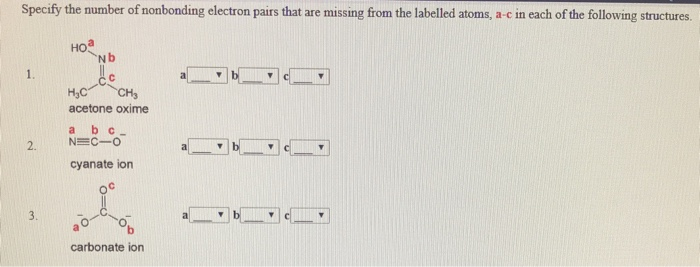
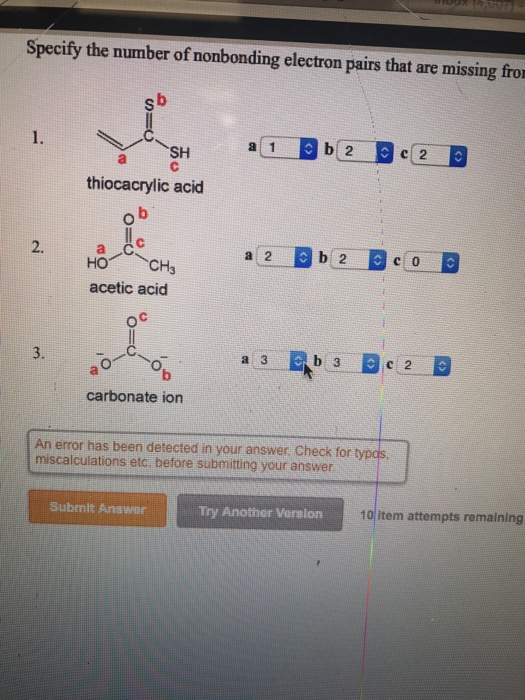
Specify The Number Of Nonbonding Electron Pairs That Chegg Com

Specify The Number Of Nonbonding Electron Pairs That Chegg Com

Specify The Number Of Nonbonding Electron Pairs That Chegg Com
Specify The Number Of Nonbonding Electron Pairs That Chegg Com

Aleks Counting Bonding And Nonbonding Electron Pairs In A Lewis Structure Youtube
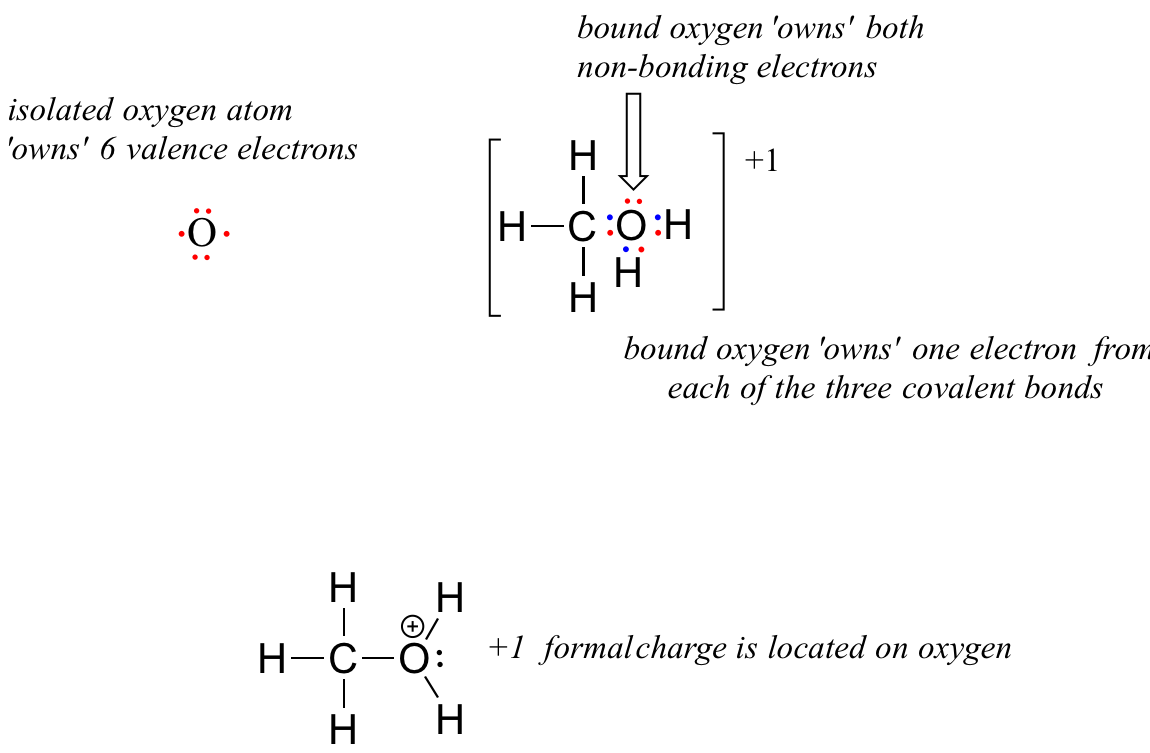
1 6 Lewis Structures Chemistry Libretexts

Solved Identify All Nonbonding Lone Pairs Of Electron In The Following 1 Answer Transtutors

9 1a Counting Bonding And Nonbonding Electron Pairs In A Lewis Structure Youtube
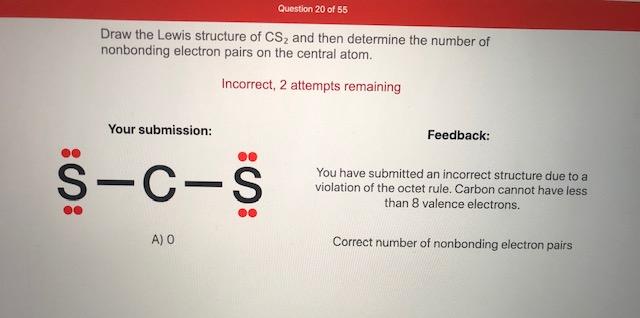
Answered Draw The Lewis Structure Of Cs And Bartleby

The Total Number Of Nonbonding Electron Pairs Present Chegg Com
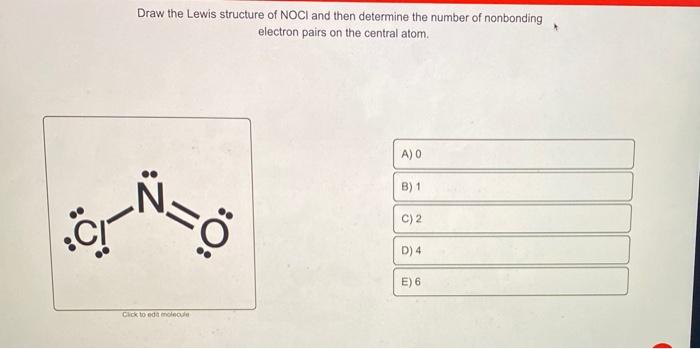
Draw The Lewis Structure Of Noci And Then Determine Chegg Com
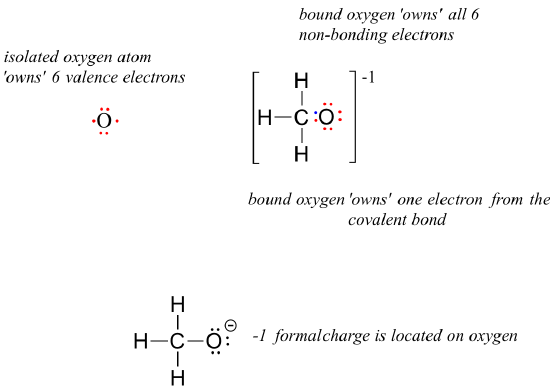
1 2 Drawing Organic Structures Chemistry Libretexts

Determine The Number Of Bonding Electrons And The Number Of Nonbonding Electrons In The Structure Of Brainly Com
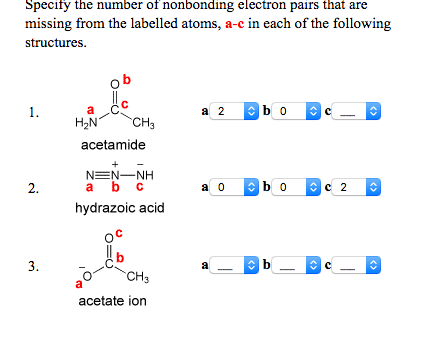
Specify The Number Of Nonbonding Electron Pairs That Chegg Com
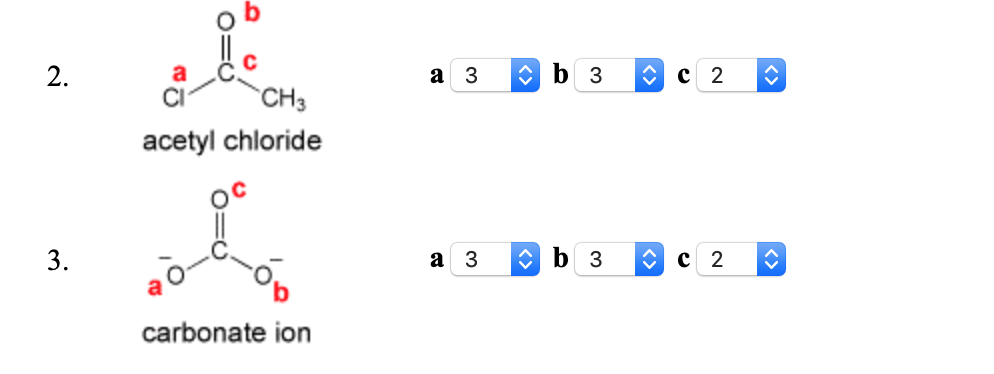
Specify The Number Of Nonbonding Electron Pairs That Chegg Com
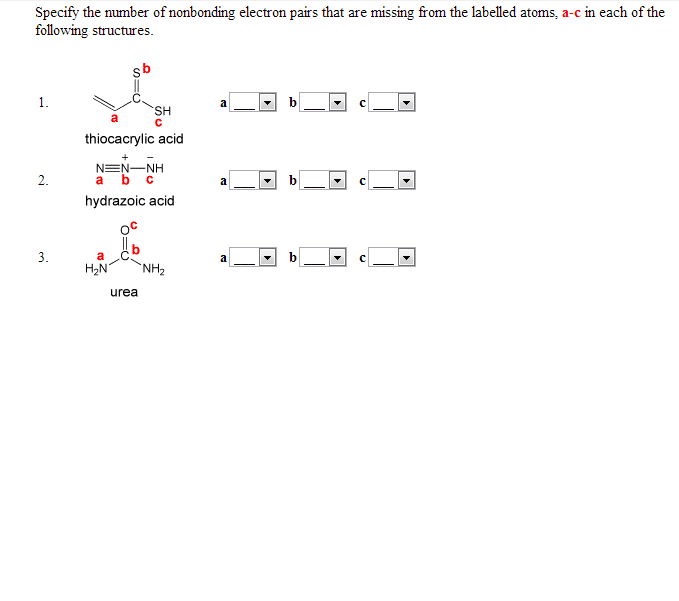
Specify The Number Of Nonbonding Electron Pairs That Chegg Com
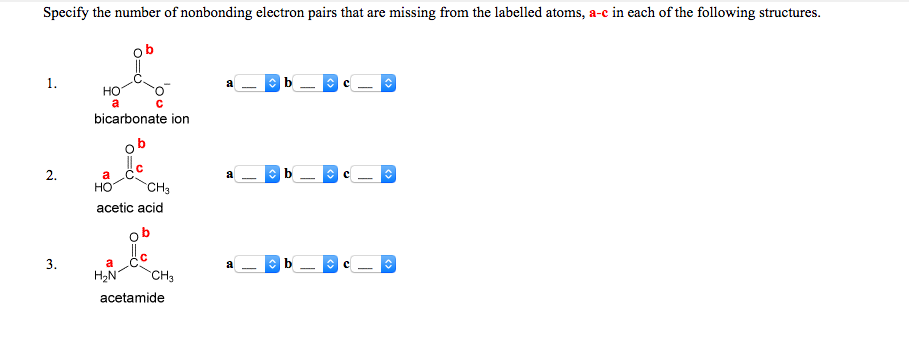
How Do You Determine The Amount Of Non Bonding Chegg Com