How To Tell Which Lewis Structure Is More Stable
Several Lewis structures are used collectively to describe the actual molecular structure which is an approximate intermediate between the canonical forms called a resonance hybrid. See the following examples for how to draw Lewis dot structures for common atoms involved in covalent bonding.

The Predicted Stabilities Of Resonance Contributors Mcc Organic Chemistry
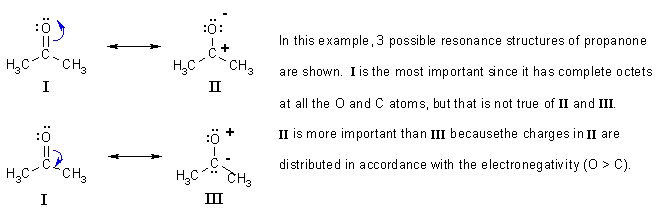
Typically the structure with the most charges on the atoms closest to zero is the more stable Lewis structure.
How to tell which lewis structure is more stable. The more stable weaker the conjugate base the stronger the acid. Contributing structures differ only in the position of electrons not in the position of nuclei. Lewis dot structures can be drawn to show the valence electrons that surround an atom itself.
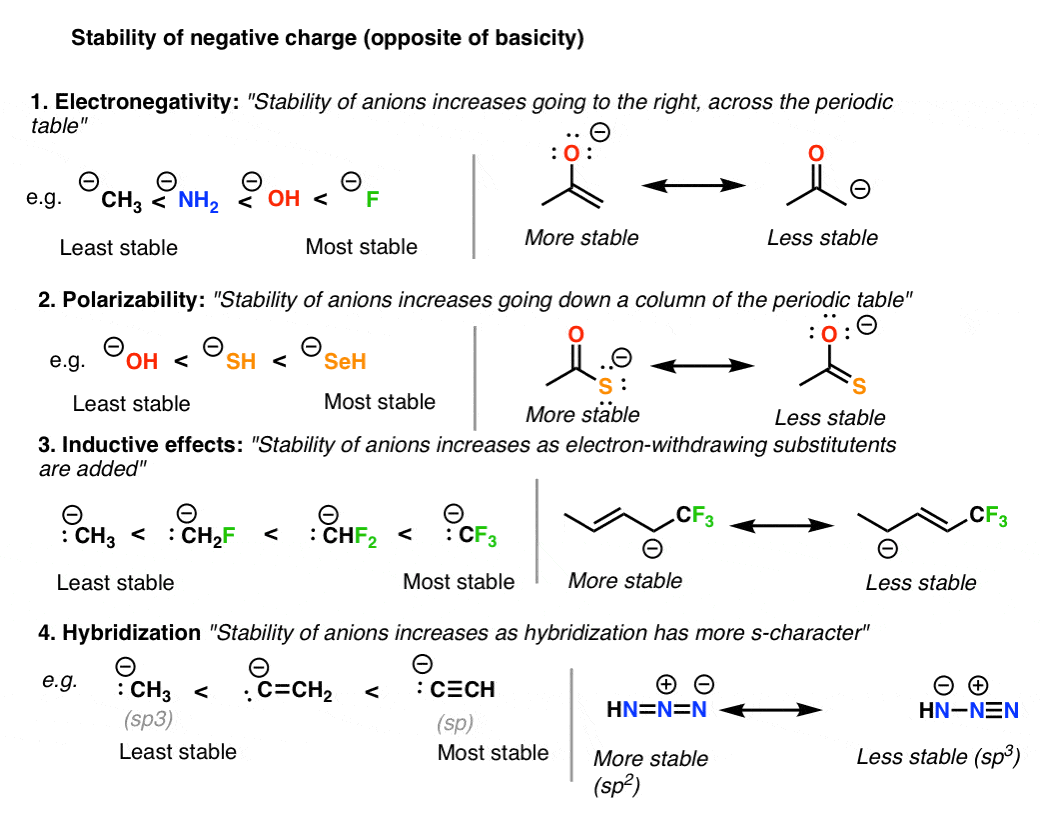
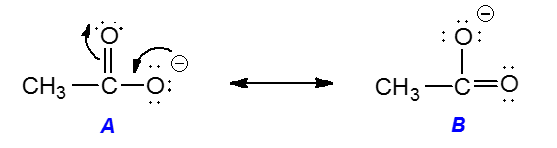
Adjacent atoms should have opposite formal charges or zero formal charge. Draw the Lewis Dot Structure for the. A structure with a negative charge on the more electronegative atom will be more stable.
This ties into resonance structures - for example if you have charges on one form of -2 1 -1 and on another form 010 the second form would be the most stable and the lowest energy. Electron delocalization lowers the potential energy of the substance and thus makes it more stable than any of the contributing structures. Yes when you are drawing lewis structures you should be evaluating your structure to determine if it is the most stable version.
The structure with the least separation of formal charge is more stable. Well its just the movement of ele. In cases where there are positive or negative formal charges on various atoms stable structures generally have negative formal charges on the more electronegative atoms and positive formal charges on the less electronegative atoms.
The resonance structure with a complete octet is more stable. In cases where there are positive or negative formal charges on various atoms stable structures generally have negative formal charges on the more electronegative atoms and positive formal charges on the less electronegative atoms. Draw Lewis dot structure for SO2.
The structure with the least number of formal charges is more stable The structure with the least separation of formal charge is more stable A structure with a negative charge on the more electronegative atom will be more stable Positive charges on the least electronegative atom most electropositive is more stable. A step-by-step explanation of how to draw the COCl2 Lewis Dot Structure PhosgeneFor the COCl2 structure use the periodic table to find the total number of. This type of Lewis dot structure is represented by an atomic symbol and a series of dots.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. The structure with the least separation of formal charges is more stable. Out of two conformations the one with lower energy is more stable.
The structure with the least number of formal charges is more stable. The structure with the least number of formal charges is more stable. While studying resonance from this Chemistry LibreTexts article I found the rules to follow in order to decide which structure is the most stable.
Positive charges on the least electronegative atom most electropositive is more stable. Calculating the formal charge is also a good tool. To choose the most stable Lewis structure with the lowest energy calculate the formal charges for all the atoms and choose the molecule that has all of them closest to zero.
Viewed 2k times. Structures with the lowest magnitude of formal charges are more stable. So despite having two axial groups the first conformer is more as two chlorines do not bring as much steric interaction as the methyl group.
Atoms in general dont like charges so having no charge is better. The key to understanding this trend is to consider the hypothetical conjugate base in each case. Look at where the negative charge ends up in each conjugate base.
More electronegative atoms should have negative formal charges. Typically the structure with the most charges on the atoms closest to zero is the more stable Lewis structure. Sometimes it is impossible to avoid charges so if both resonance structures are charged then the octet rule needs to be considered.
Need help with chemistry. Are you struggling with Resonance structures or just dont really get whats going on when you do it. Following the rules for drawing lewis structures should help guide you in determining this.

N2o4 Dinitrogen Tetroxide Resonance Structures

The Predicted Stabilities Of Resonance Contributors Mcc Organic Chemistry

The Predicted Stabilities Of Resonance Contributors Mcc Organic Chemistry

How To Choose The More Stable Resonance Structure Chemistry Steps

How To Choose The More Stable Resonance Structure Chemistry Steps
Resonance Structures 4 Rules On How To Evaluate Them With Practice
1 5 Resonance Structures Dat Bootcamp
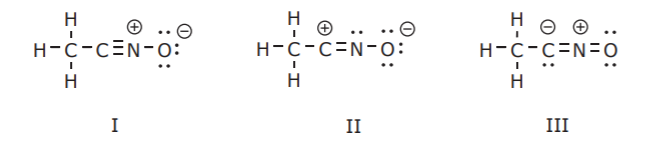
4 3 Resonance Structures Chemistry Libretexts
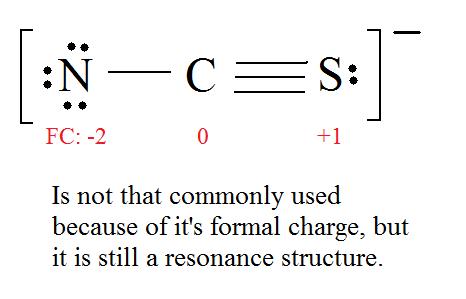
The Predicted Stabilities Of Resonance Contributors Mcc Organic Chemistry
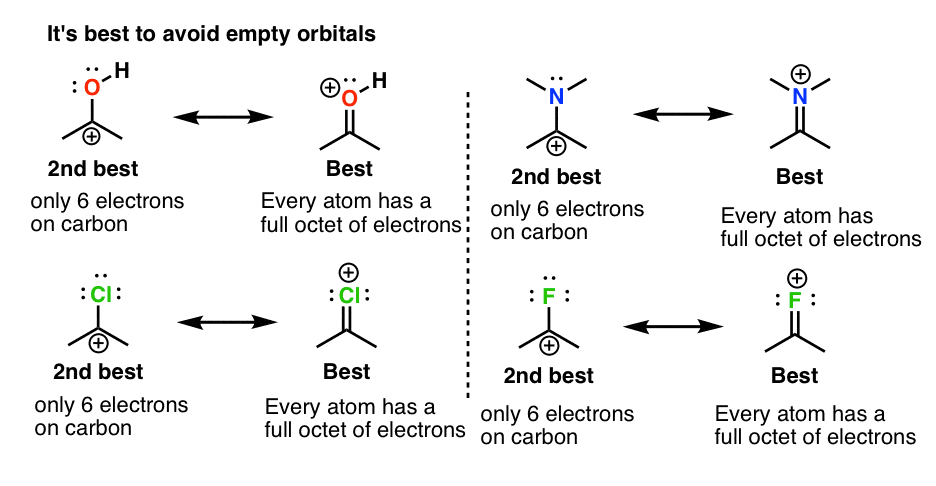
Evaluating Resonance Structures With Positive Charge

The Predicted Stabilities Of Resonance Contributors Mcc Organic Chemistry

How To Choose The More Stable Resonance Structure Chemistry Steps

How To Choose The More Stable Resonance Structure Chemistry Steps

The Predicted Stabilities Of Resonance Contributors Mcc Organic Chemistry

How To Choose The More Stable Resonance Structure Chemistry Steps

The Predicted Stabilities Of Resonance Contributors Mcc Organic Chemistry

How To Choose The More Stable Resonance Structure Chemistry Steps
