Is C2h4 Lewis Acid
C2H4 is a neutral compoundSo it has zero charge or no chargeH-CC-HIt is etheneDouble bond referring eneIt is covalent bond which means mutual sharing of bon 1. Write Clearly and Concisely Grammarly.
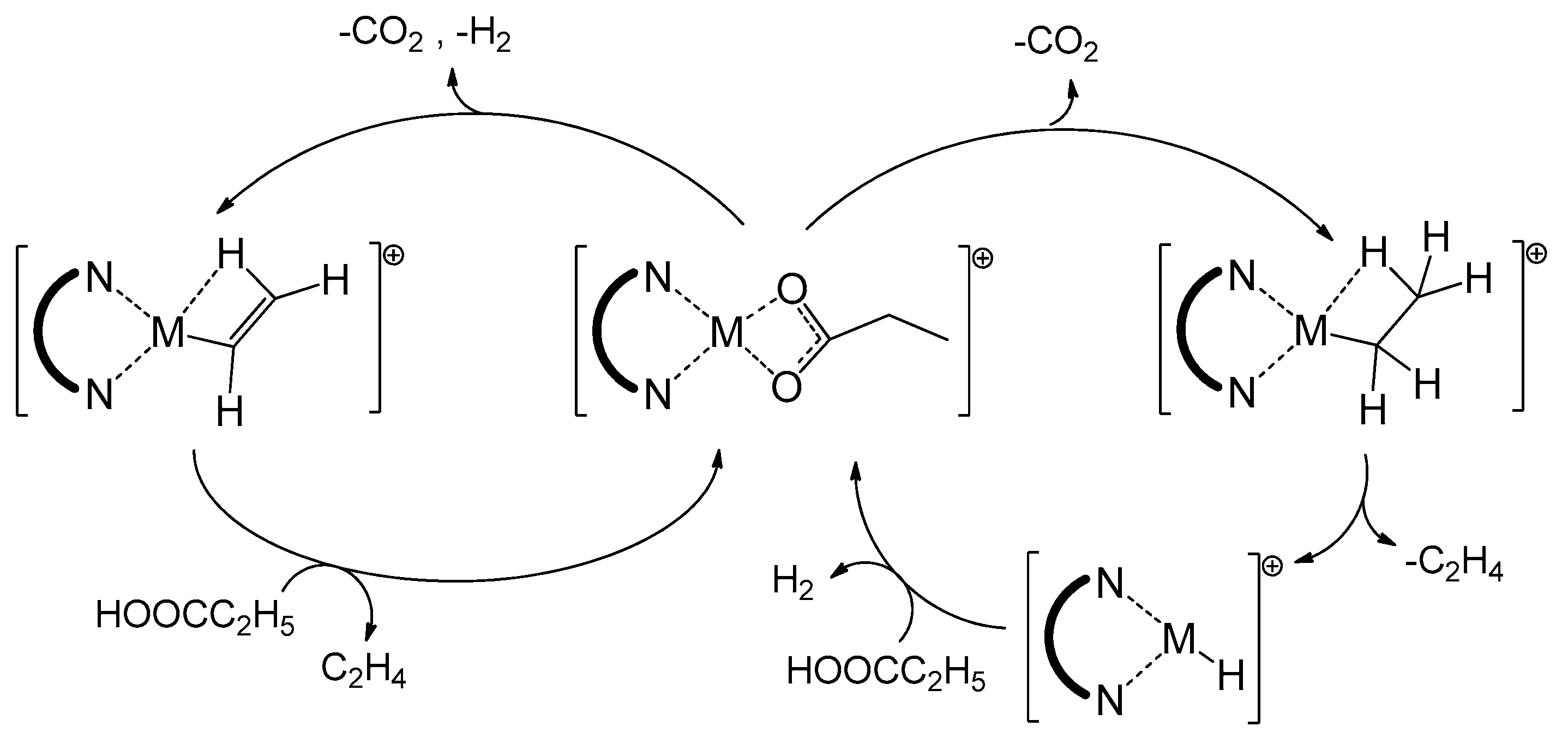
Reactions Free Full Text Mechanism Of Deoxygenation And Cracking Of Fatty Acids By Gas Phase Cationic Complexes Of Ni Pd And Pt Html
The correct option is.

Is c2h4 lewis acid. Carbon monoxide is an excellent ligand towards low valent transition metals. C 2H 4 is CH 2CH 2 it has one double bond that means it has pi electrons. Lewis acids and bases are described by the Lewis theory of acid-base reactions as electron-pair acceptors and electron pair donors respectively.
C2H4 is not a Lewis acid because the molecules in BF3 and FeCl3 in the central atom have an incomplete octet. It is a chemical formula for Ethylene or Ethene. Given the triple bond there is a formal negative charge on the carbon and a formal positive charge on the oxygen.
Hence according to Lewis concept these are Lewis acids. C2H4 Lewis Structure Ethylene Welcome to Geometry of Molecules and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure of C2H4 molecule. CH4 methane is lewis base What is an acid base neutral.
Log Octanol-Water Partition Coef SRC. When carbon monoxide binds to a transition metal centre. You can eliminate the.
C2H4 Lewis Dot Structure - How to Draw the Lewis Structure for C2H4 - YouTube. H2o neutral water Ch3oh none methanol Koh strong base potassium hydroxide Cscl salt cesium chloride H2so4 strong acid sulfuric acid C12h22o11 none sucrose. The correct option is.
Therefore ion act as Lewis acid as it accepts electron pairs from the oxalate ion which is a Lewis base. Hydrochloric acid cannot be classified as a Lewis acid since it cannot accept an electron pair. Is C2H4 a Lewis acid.
C2h4 is a lewis base because because it has sufficient elecctron pair and can therefore donte electrons in a covalent bond. C 2H 4 is CH 2CH 2 it has one double bond that means it has pi electrons. Ethene or ethylene C2H4 is not a Lewis acid.
However this compound dissociates into its constituent ions liberating H ions which are considered as Lewis acids. SiF4 can act as a Lewis acid because Si can expand. ROH is a Lewis base because it has an lone pair of electron.
So it works as a Lewis acid. Hence according to Lewis concept these are Lewis acids. Due to its inability to accept electron pairs hydrochloric acid is often referred to as a classical acid rather than a Lewis acid.
C2h4 is a lewis base because because it has sufficient elecctron pair and can therefore donte electrons in a covalent bond. Is Roh a Lewis acid or base. Lewis Dot Structure of CH3COOH Acetic Acid Watch later.
Recategorized Feb 11 2020 by subrita. It is demonstrably an electron pair acceptor. Is sicl4 a Lewis acid.
In the case of a π-acceptor ligand the acceptor orbitals are unoccupied which is why the ligand can serve as an acceptor and the resulting π orbitals are also unoccupied. Why is SiF4 a Lewis acid. Is c2h2 a Lewis acid.
Log Kow KOWWIN v167 estimate 127 Log Kow Exper. Now sicl4 is able to keep greater than 8 electrons in its outermost orbit. Which of the following is not a Lewis acid.
Why C2H4 is not a Lewis acid. Sui H Zhang F Hou F1. If playback doesnt begin shortly try restarting your device.
Predicted data is generated using the US Environmental Protection Agencys EPISuite. The SiF4 in the central atom and according to Lewiss concept these three are Lewis acid. B C2H4 ExplanationIn BF3 and FeCl3 molecules the central atoms have incomplete octet and in SiF4 the central atom has empty d-orbitals.
Carbon monoxide is in fact a potent Lewis-acid. Database match 113 Exper. So it takes electron pairs from other compounds which have more electron density.
B C2H4 ExplanationIn BF3 and FeCl3 molecules the central atoms have incomplete octet and in SiF4 the central atom has empty d-orbitals. The usual Lewis formulation is C-O. C2H4 is a Lewis base due to availability of electons as a pi bond.
Acetic acid is not a Lewis acid because it cannot form a covalent bond with an electron pair. It can also increase its coordination number.

Hybridisation Of C2h4 Sp2 Hybridisation Formation Of Ethene Molecule Youtube

Comparative Study Of 1 1 Lewis Acid Base Adducts Between Cp2m L H M V Nb Ta L Co C2h4 P Ch3 3 And Bf3 Alf3 Semantic Scholar

Molecular Graphs Of Electron Density L Co A C2h4 B P Ch3 3 Download Scientific Diagram

Draw The Electron Dot Structure Of Ethene C2h4 Brainly In

Molecular Graphs Of Electron Density L Co A C2h4 B P Ch3 3 Download Scientific Diagram

Structures Of The C2h2 Hf I C2h4 Hf Ii And C3h6 Hf Iii Download Scientific Diagram
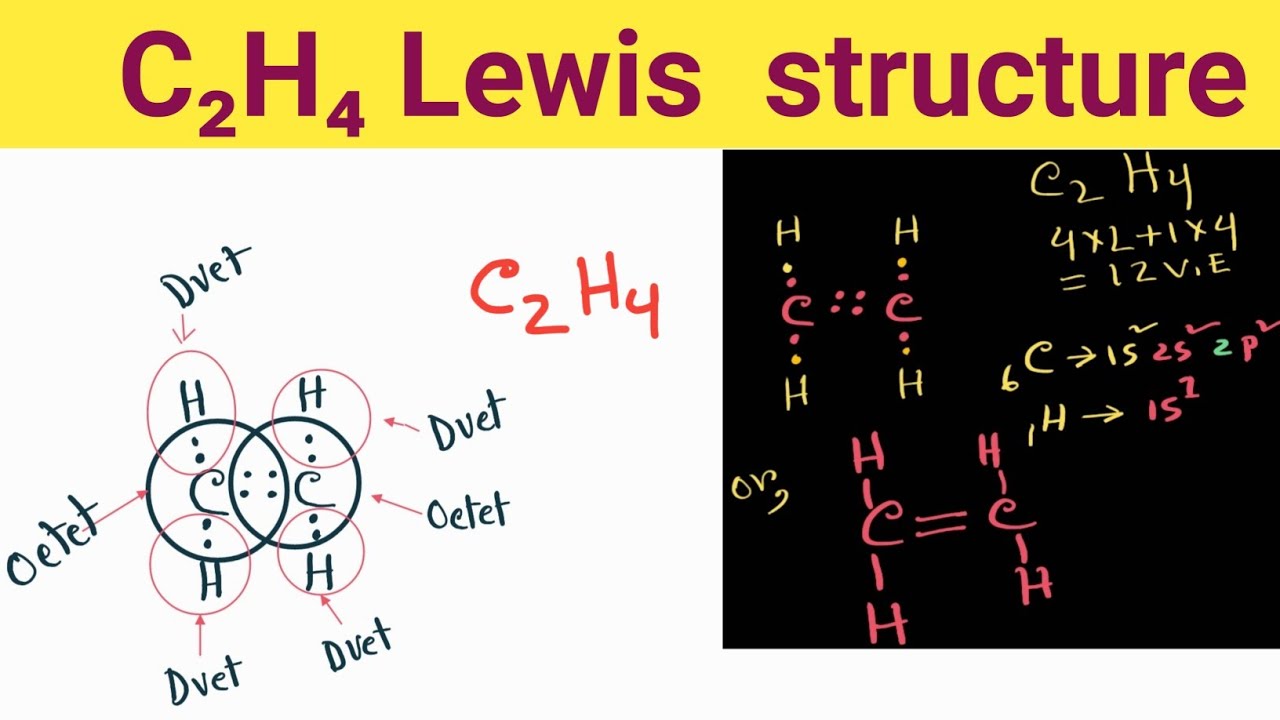
C2h4 Lewis Structure How Do You Draw The Lewis Structure For C2h4 Youtube
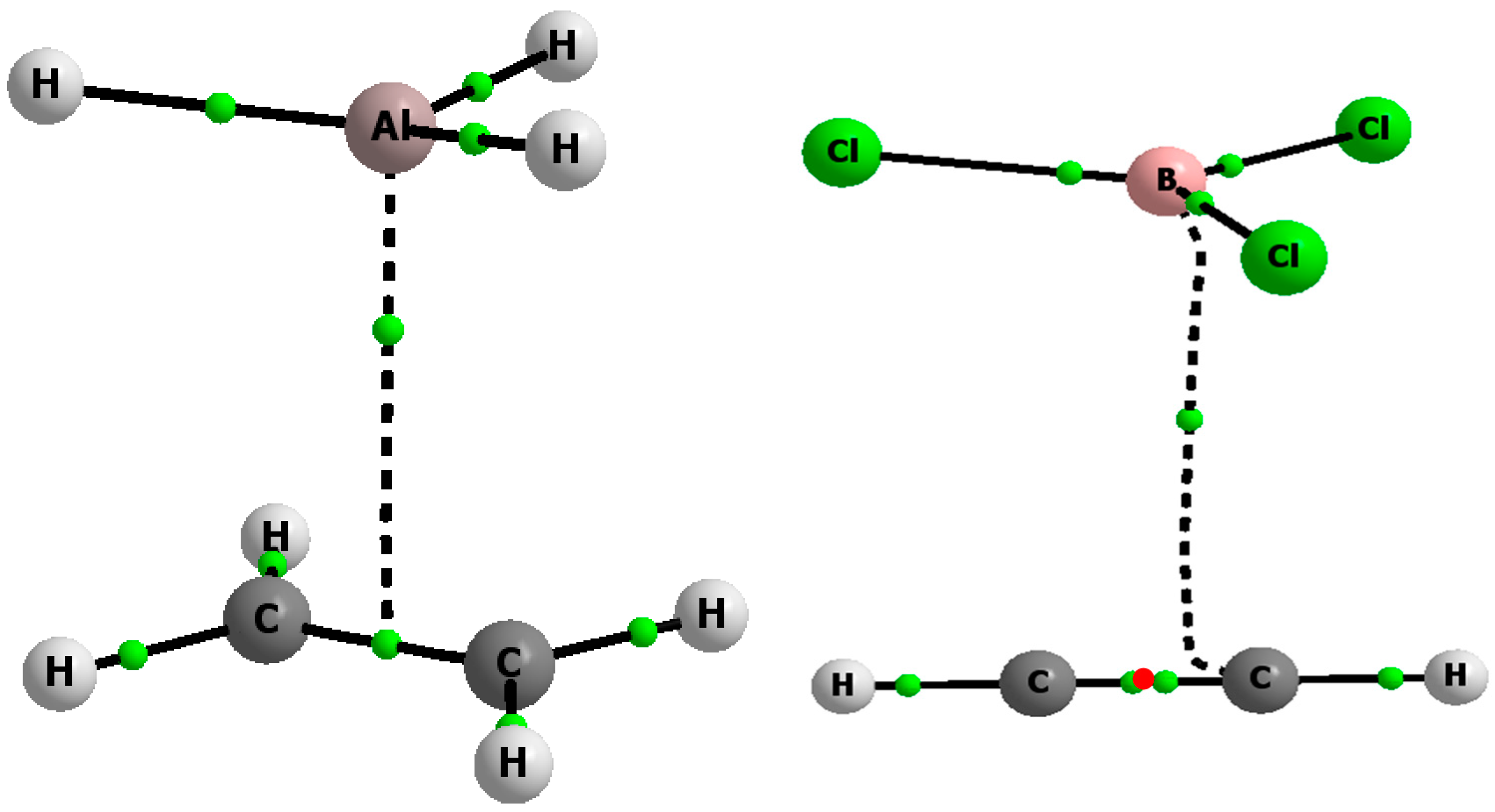
Molecules Free Full Text Triel Bonds P Hole P Electrons Interactions In Complexes Of Boron And Aluminium Trihalides And Trihydrides With Acetylene And Ethylene Html

Experimental Microwave Mw Structure Of Complex C2h4 Ag Cl Download Scientific Diagram

The Molecular Graphs Of The Alf3 C2h2 The Top Left Albr3 C2h4 The Download Scientific Diagram

Is C2h4 Polar Or Nonpolar All About C2h4 Polarity
C2h4 Resonance Structure Shefalitayal

By 107 Which Of The Following Is Not A Lewis Acid Aipmt 1996 A Sif4 B C2h4 C Bf3 D Fecl3

Which Of The Following Is Not A Lewis Acid A Sif4 B C2h4 C Bf3 D F

Which Of The Following Is Not A Lewis Acid A Sif4 B C2h4 C Bf3 D F

Esters Chemistry Lessons Chemistry Math Equations

What Are Hydrocarbons Gulf Coast Environmental Systems Chemistry Organic Chemistry Chemistry Classroom
