Is H2so4 Lewis Acid
Properties of HSO4-Sodium Bisulfate NaHS04 which is the pure substance starts melting at 185C and begins to lose water and forms the hydrogen sulphate ion HSO4. Is H2SO4 Lewis acid.

Draw And Explain The Lewis Structure For Sulfuric Acid Study Com
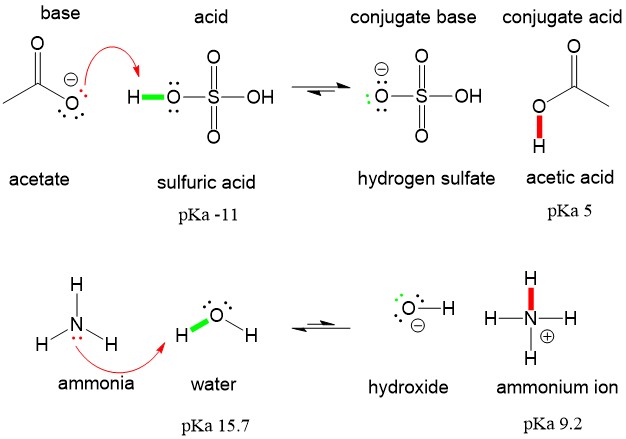
However the conjugate base of sulfuric acid bisulfate anion is itself a reasonably strong Bronsted acid.

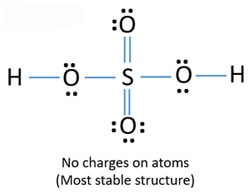
Is h2so4 lewis acid. A Lewis acid is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. When we have an H or H2 in front of a polyatomic molecule like CO3. Therefore there should be two -OH groups in H2SO4 molecule.
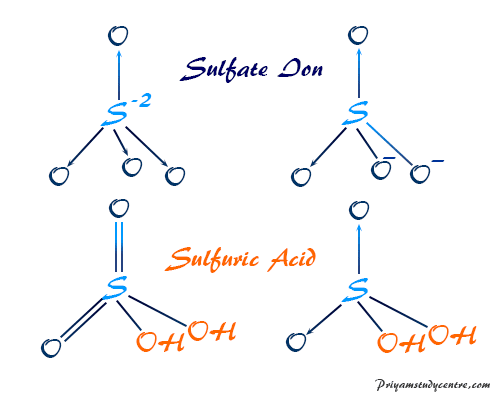
Because sulfuric acid is a dibasic acid it can release two H ions in the water. A sufficiently low pH is genotoxic to some cell systems. Schwefelsäure ist eine chemische Verbindung des Schwefels mit der Summenformel H 2 SO 4Sie ist eine farblose ölige sehr viskose und hygroskopische FlüssigkeitSchwefelsäure ist eine der stärksten Säuren und wirkt stark ätzendDiese Mineralsäure bildet zwei Reihen von Salzen die Hydrogensulfate und die Sulfate bei denen im Vergleich zur freien Säure ein beziehungsweise zwei.
H 2SO4aq H 2Ol H SO 4 H 3O. Base is a substance which can donate a pair of electrons to form a co-ordiante covalent bond. ALL bronsted acids are lewis acids but not the other way around.
H2SO4 SULFURIC ACID is strong acid What is an acid base neutral. A step-by-step explanation of how to draw the H2SO4 Lewis Structure Sulfuric Acid. Lewis Concept of Acid and Bases.
Lewis dot of the sulfuric acid. In other words a Lewis acid is an electron-pair acceptor. This reaction goes to completion in water.
Sulfuric acid aerosol toxicity is dependent on the hydrogen ion content of the aerosol. An acid is a substance molecule or ion which can accept a pair of electrons. Van der Waals dispersion forces dipole-dipole.
Chem 1090 Lewis 8b. Lewis Dot Structure Of H2so4 Sulfuric Acid Youtube. It is classified as a weak acid as it partially dissociates to form H and SO42-.
Sulfuric acid H 2 SO 4. A step by step explanation of how to draw the h2so4 lewis structure. Lewis structure of sulfuric acid is drawn step by step in this tutorial.
S does not follow the octet rule. A Lewis base is therefore an electron-pair donor. H2So4 Lewis.
H2So4 is a lewis acid because it is a bronsted acid. I also go over hybridization shape and bond angles. A Lewis structure for the oxyacid sulfuric acid.
It has a molecular weight of 98079 gmol. H 2 so 4 step 7 picture so far. For example NH3 is a Lewis base because it can donate its lone pair of electrons.
Therefore we can assume there should be two -OH bonds in sulfuric acid molecule. Hence this is the most stable Lewis structure for HSO4-. H Ions HCl BF3 etc.
E sulphuric acid H2SO4 bonded as. Ethylene C2H4 has the Lewis Structure. There is evidence that pH plays a critical role in growth control and cell differentiation and that disrupting the control of pH may lead to adverse effects.
A Lewis base then is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. Lewis structure of sulfuric acid is drawn in this tutorial step by step. Sulfuric acid is a covalent compound as all of the bonds are covalent.
Total valence electrons concept is used to draw the lewis structure of H 2 SO 4. I quickly take you through how to draw the lewis structure of h2so4 sulfuric acid. ALL bronsted acids are lewis acids.
Is H2SO4 a hydrogen bond. The molecular shape is predicted to be trigonal planar around each carbon atom. H SO 4 H 2O SO2 4 H 3O.
En este vídeo comento los pasos necesarios para escribir la estructura de lewis del ácido sulfúrico h_2so_4. H2So4 is a lewis acid because it is a bronsted acid. Sulfuric acid can supply up to 2 H ions per molecule and H ions are Lewis Acids.
One mechanism by which sulfuric acid may produce its toxicity is by changing extracellular and intracellular pH. It means it can release two hydrogen atoms to show acidic characteristics. Sulfuric acid is a strong dibasic acid.
Therefore a significant change in pH produced by sulfuric acid. Total valence electrons concept is used to draw the lewis structure of h2so4sulfur is the central atom in h2so4. A Lewis base is any substance such as the OH- ion that can donate a pair of nonbonding electrons.
For H2SO4 molecule sulfur has the highest valence than oxygen and hydrogen. Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing When we have an h or h2 in front of a polyatomic molecule like co3. Is Sulphuric acid ionic or covalent.
Almost all simple cations which have an empty orbital in their outermost shell accept a pair of electrons from other molecules and function as Lewis acids. The answer is D. This is composed of a σ framework and a π-bond.
In the h2so4 structure lewis sera is the least electronic atom and goes to the center of lewiss structure. Keep these important points in mind while guessing Lewis acids or bases. A Lewis acid is any substance such as the H ion that can accept a pair of nonbonding electrons.
ALL bronsted acids are lewis acids but not the other way around. This means that the hydrogen atoms will be attached to. Sulfuric acid is a dibasic strong acid.
The structure is similar to that of methane. Trimethylborane is a Lewis acid. With two bonding pairs and two lone pairs the oxygen atom has now completed its explain why the following lewis.
Transcribed image text from this question. Overall H 2SO4aq 2H 2Ol 2H 3O SO2 4. The answer is D.

New Page 2 731x498 Jpeg Organic Chemistry Organic Chemistry Reactions Chemistry
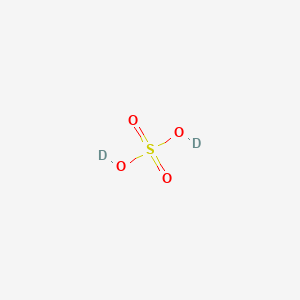
Sulfuric Acid D2 H2o4s Pubchem

H2so4 Lewis Structure Sulfuric Acid Youtube

What Is Difference Between Hydrochloric Acid And Sulfuric Acid Quora

Pin On Chemistry What Is Chemistry

A Reaction Map Pdf For Benzene And Aromatic Compounds Organic Chemistry Reactions Organic Chemistry Reactions
Sulfuric Acid H2o4s Chemspider
Sulfuric Acid H2so4 Structure Production Properties Uses
Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing

Baylis Hillman Reaction Reactions Phosphine Reaction Rate

File Sulfuric Acid Lewis Png Wikimedia Commons

Non Aqueous Solvents Nh3 Hf H2so4 N2o4 Pocl3 Socl2 Brf3 In 2021 Solvents Chemistry Agno
Reviewing Acid Base Definitions Organic Chemistry Help

Tetryonics 56 04 Common Acids Are Formed Where Protons Bind To Chemical Elements And Compounds To Create Incomplete Atomic Orbitals And Charged Cations
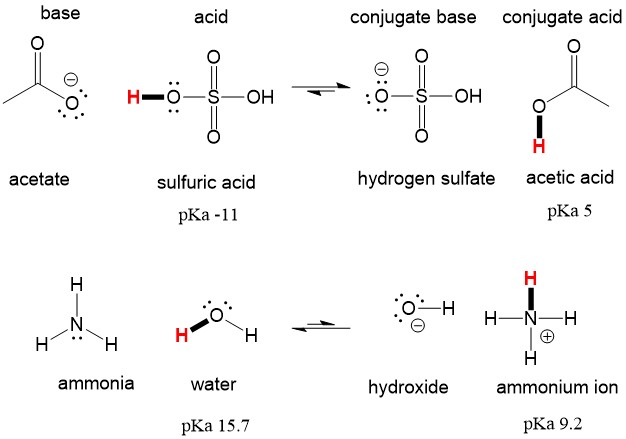
Reviewing Acid Base Definitions Organic Chemistry Help