Is Pf5 A Lewis Acid
How can these relative reactivities as a Lewis acids be rationalized. Toxic and corrosive fumes are generated when this material is.
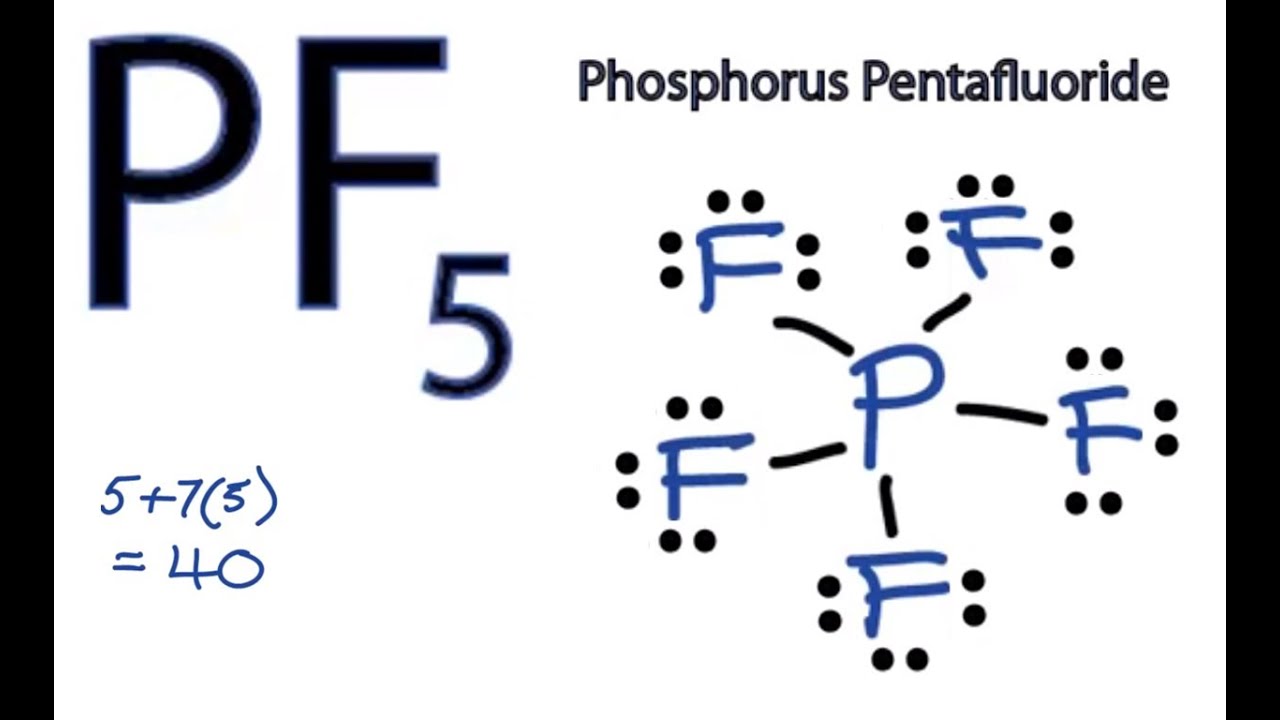
Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube
Include geometry andformal charges in the structures.
Is pf5 a lewis acid. Which of the following is a Lewis acid. The Lewis acidity should be largest for PF3 since the electronegative fluorine atoms would reduce electron density of the phosphorus atom and make it more hungry for electrons. In other words a Lewis acid is an electron-pair acceptor.
Hope that helps and forgive me for grammar and spelling errors - I am. It is very toxic by inhalation and can cause pulmonary edema. CeSbF5 AsF5 PF5 One simple argument could be the size of the ceSb-center.
For polyatomic species consider the Lewis acid behavior of the central atom. I couldnt find a more precise explanation or rationalisation. Lewis acid is therefore any substance such as the H ion that can accept a pair of nonbonding electrons.
A Lewis acid is a species that can accept an electron pair whereas a Lewis base has an electron pair available for donation to a Lewis acid. Because it is bigger than for example a ceP-center it can accept more ligands. What is the adduct that isformed Include a picture if you can.
Draw molecular geometries for the reactants and the product ofthe reaction of PF5 with the Lewis base NH3. Select the correct answer below. Although it is only weakly basic SbF 6 does react with additional SbF 5 to give a centrosymmetric adduct.
Therefore it is considered as a Lewis acid. Es ist bei Standardbedingungen ein farbloses sehr giftiges und nicht brennbares Gas mit stechendem Geruch. SbF 5 is a strong Lewis acid exceptionally so toward sources of F to give the very stable anion SbF 6 called hexafluoroantimonate.
My knowledge of the MO diagram of hypercoordinative compounds is really basic so. Moreover the fluorines small size allows more space for extra bonding. Phosphorus pentafluoride is a colorless poisonous nonflammable compressed gas with a pungent odor.
All cations are Lewis acids since they are able to accept electrons. In a complex ion we have a central atom often consisting of a transition metal cation which acts as a Lewis acid and several neutral molecules or ions surrounding them called ligands that act as Lewis bases. Complex ions are examples of Lewis acid-base adducts.
The phosphorus in PCl5 readily accepts electrons from other molecules. I believe that thats correct where Arrhenius and Bronsted is a reaction with water but I believe that lewis acids and bases dont necessarily have to be in water. Phosphorpentafluorid Phosphorpentafluorid Phosphor V-fluorid ist eine anorganische chemische Verbindung aus den Elementen Phosphor und Fluor mit der Summenformel PF 5 und zählt zur Verbindungsklasse der Phosphorhalogenide.
Lone pair-lone pair repulsion would make backbonding even less favourable. It is extremely irritating to skin eyes and mucus membranes. Which compound will be a stronger Lewis acid PF5 or PCl5Explain briefly.
SbF 6 is a weakly coordinating anion akin to PF 6. According to the Lewis concept acid is the substance that accepts a lone pair of electrons since it has empty orbitals in the valence shell. For example PF5 F- Pf6 is a lewis acid-base reaction.
3 question Phosphorus pentafluoride PF5 acts as a during the formation of the anion PF6. The answer to the second question is again yes they can both behave as Lewis acids again because of expanded octets and accepting electron pairs. Upvote 0 Downvote Add comment.

Writing Lewis Structures Ppt Download

An Electrolyte Additive Capable Of Scavenging Hf And Pf5 Enables Fast Charging Of Lithium Ion Batteries In Lipf6 Based Electrolytes Sciencedirect

1 8 Chemistry 2810 Answers To Assignment 3 Topic Lewis

Is Pf5 Polar Or Nonpolar Techiescientist
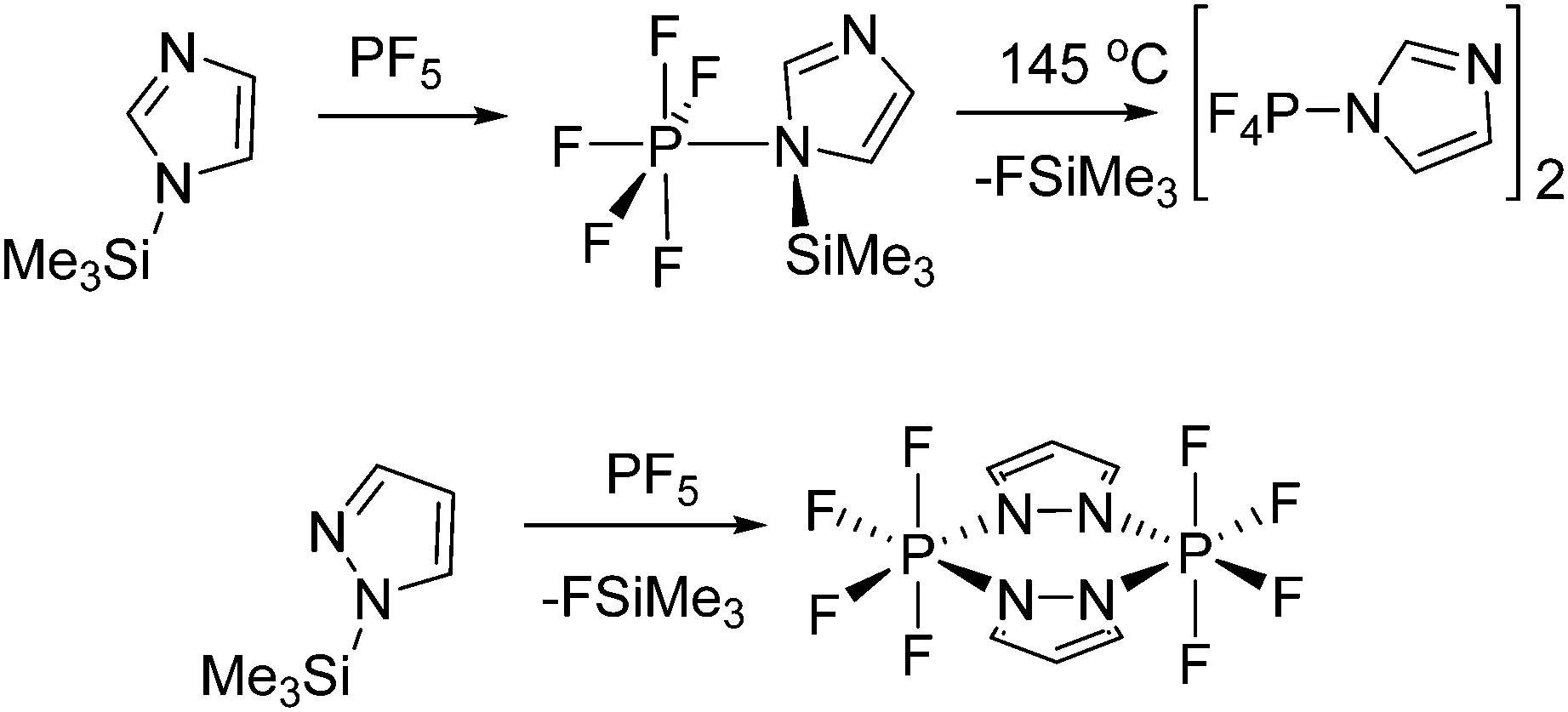
Phosphorus Lewis Acids Emerging Reactivity And Applications In Catalysis Chemical Society Reviews Rsc Publishing Doi 10 1039 C5cs00516g
Http Www Carbene De Wp Content Cm 1111 Tutorial 6 With Answers Pdf

Pf5 Lewis Structure And Molecular Geometry Made Easy Youtube
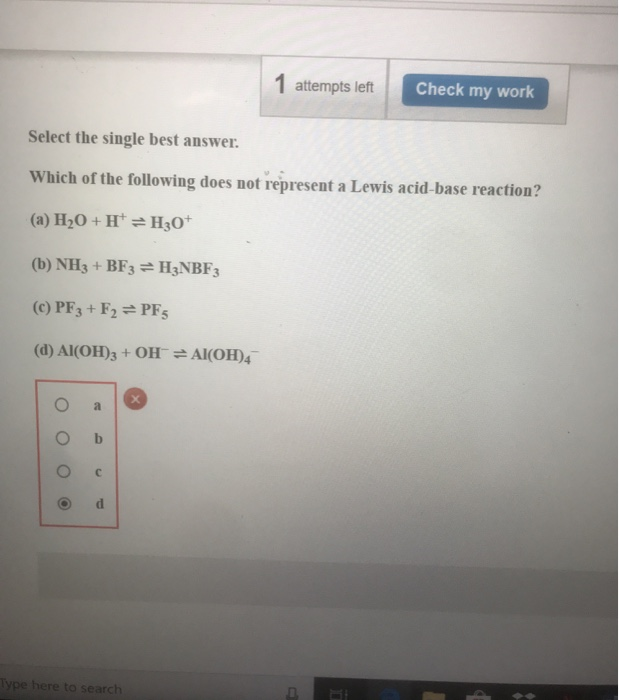
1 Attempts Left Check My Work Select The Single Best Chegg Com
Welcome To Chem Zipper Com Lewis Acid Base Concept

Pf5 Lewis Structure Phosphorus Pentafluoride Youtube

Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube
Solved The Uorides Bf3 Aif3 Sif4 And Pf5 Are Lewis Acids The All Form Very Stable Uoroanions When Treated With Lithium Uoride In Contrast The Course Hero

Possible Mechanism For The Ec Decomposition Via Strong Lewis Acid Pf Download Scientific Diagram
Https Www Tandfonline Com Doi Pdf 10 1080 15533179208020275

Lewis Acid Catalyzed Synthesis Of Cyanidophosphates Blasing 2016 Chemistry A European Journal Wiley Online Library
Solved The Uorides Bf3 Aif3 Sif4 And Pf5 Are Lewis Acids The All Form Very Stable Uoroanions When Treated With Lithium Uoride In Contrast The Course Hero
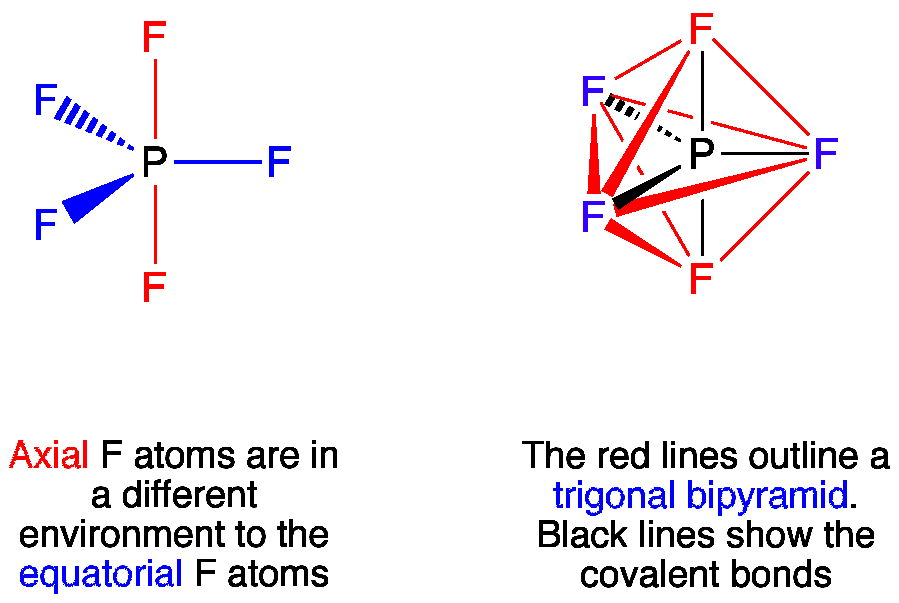
Vsepr Pf5 Phosphorus Pentafluoride
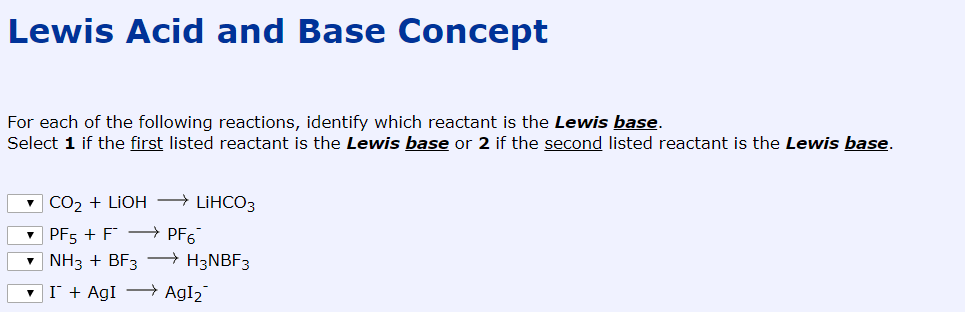
Lewis Acid And Base Concept For Each Of The Following Chegg Com
