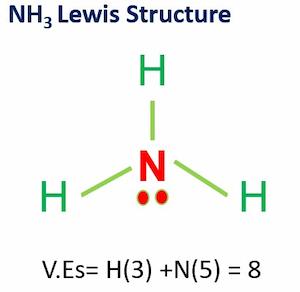
Lewis Dot Structure For Nh3
CH3I Lewis dot Structure by counting valence electrons on carbon atom. Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia NH3 would be three hydrogen atoms bonded to a nitrogen atom in the middle with a lone pair of electrons.

Give The Electron Dot Structure For Ammonia Nh3 Study Com
2NH3 are two molecules of NH3 the Lewis structure for NH3 has three Hydrogens sticking out of NitrogenDont forget the lone pair electrons in N.

Lewis dot structure for nh3. The VSEPR Model of Molecular Geometry-Ronald J Gillespie 2013-03-21 Authoritative reference features extensive coverage of structural information as well as theory and applications. The Lewis structure of ammonia NH3 would be three hydrogen atoms bonded to a nitrogen atom in the middle with a lone pair of electrons. The Lewis structure of ammonia NH_3 would be three hydrogen atoms bonded to a nitrogen atom in the middle with a lone pair of electrons on top of the atom.
Compare h2o nh3 and ch2cl2. The Lewis dot structures are drawn for you on page 12 of your Chapter 4 Pt. As per the NCl3 lewis dot structure nitrogen is the central atom that has 3 bonded pairs of electrons and one lone present on it.
How_to_draw_lewis_dot_structure_for_nh3 414 How To Draw Lewis Dot Structure For Nh3 concepts in the context of everyday life. The structure of alanine is. The Lewis structure of the tetra atomic ammonia NH3 molecule has three single sigma bonds between the nitrogen and the hydrogen atoms.
NF3 is polar in nature. To sketch the Lewis structure of CH3I by following these instructions. Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia NH3 would be three hydrogen atoms bonded to a nitrogen atom in the middle with a lone pair of electrons.
Nh3 lewis structure 3d model. The central atom of this molecule is carbon. Nh3 lewis structure 3d model.
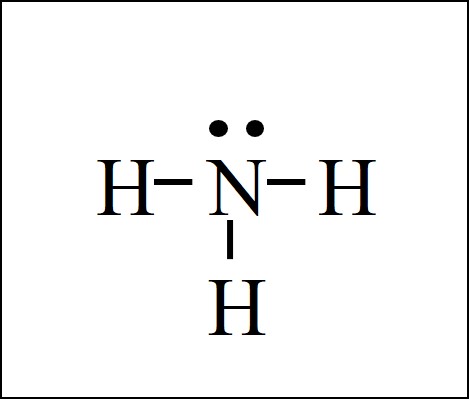
Electron Dot Structure of NH3 by Jeff Bradbury - February 17 - Lewis Electron Dot Structure for ammonia molecule NH3. Nh3 lewis dot diagram structure molecular geometry shape bond alqurumresort angle. NH3 Lewis Structure In Lewis structureit is common that a bonding pair of two electrons can be shown by dash - or dots but a lone pair of two electrons is shown by dots.
How_to_do_lewis_dot_structure_for_nh3 48 How To Do Lewis Dot Structure For Nh3 up the basics of any skill in record time. Hutchinson 2009-09-01 Organic Chemistry Volume 1-Roger Macomber 1996-04-26 This two-volume set is designed for courses focused on the fundamentals of. There are 8 valence electrons available for the Lewis structure for NH 3.
Hybridization of NF3 is Sp³. Since it is bonded to only one carbon atom it must form a double bond. The H C Calculating NH3 Formal Charges.
Moreover the presence of a single lone pair of electrons on the nitrogen atom is responsible for the bent geometrical structure of the NH3 molecule. H o h h h structural formula ball and stick. Lewis Dot Structure Of Nh3 Exam 3.
This is the reason why ammonia acts as a Lewis base as it can donate those electrons. Ammonia or NH3 has a total of 8 valence electrons. The molecular geometryVSEPR shape of NF3 is a trigonal pyramid and its electron geometry is tetrahedral.
Oxygen contains 6 valence electrons which form 2 lone pairs. The Lewis electron dot structures of a few molecules are illustrated in this subsection. How_to_draw_a_lewis_dot_structure_for_nh3 313 How To Draw A Lewis Dot Structure For Nh3 may not be available in the ebook version.
It is a pictorial representation of the arrangement of valence electrons around the individual atoms in the molecule. Lewis Structure Examples. Hence the formula of NCl3 becomes AX 3 N 1 So according to the VSEPR chart if the molecule has the formula of AX 3 N 1 then the molecule shape of that molecule is trigonal pyramidal and electron geometry is tetrahedral.
There are 8 valence electrons available for the lewis structure for nh 3. Lewis nh3 dot structure ammonia electron diagram nh trigonal planar structures pyramidal bond polarity geometry hydrogen. Ammonia nh 3 lewis and three dimensional structures.
The total valence electron available for the Nitrogen trifluoride lewis structure is 26. The lewis dot structure for nh3 ammonia is shown above. Osmoregulation in the cartilaginous fishes draw electron dot structure of co2 and NH3 Brainly in Which of the following has the smaller bond angle.
NH3 Lewis Structure The Lewis structure of a molecule helps understand the electron geometry molecular geometry polarity and other such properties with ease. Dot lewis nh3 structure electron diagram structures chemistry drawing tutorvista draw formula nh synthonix hydrogen octet each examples. And have more fun along the way.
To calculate the valence electron of each atom in CH3I look for its periodic group from the periodic table. Lewis Structure of CO2. In NH3all hydrogens follows the duet rule and the nitrogen atom follows the octet rule.
Concept Development Studies in Chemistry-John S. This integrated approach encourages curiosity and demonstrates the relevance of chemistry and its uses in students lives their future careers and their world.
Nh3 Lewis Structure Molecular Geometry What S Insight
Lewis Dot Structure Easy Hard Science
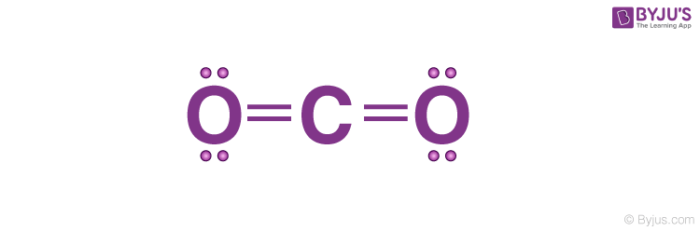
Lewis Electron Dot Structures Detailed Explanation With Examples Videos

What Is The Lewis Structure Of Nh3 Study Com

Dot Structure Of Nh3 Brainly In
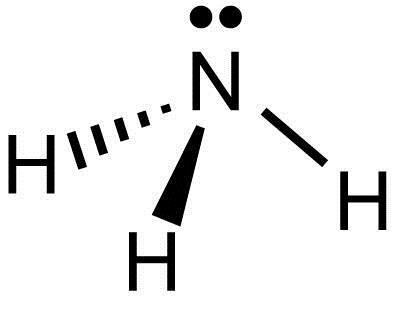
What Is The Lewis Structure Of Nh3 Socratic
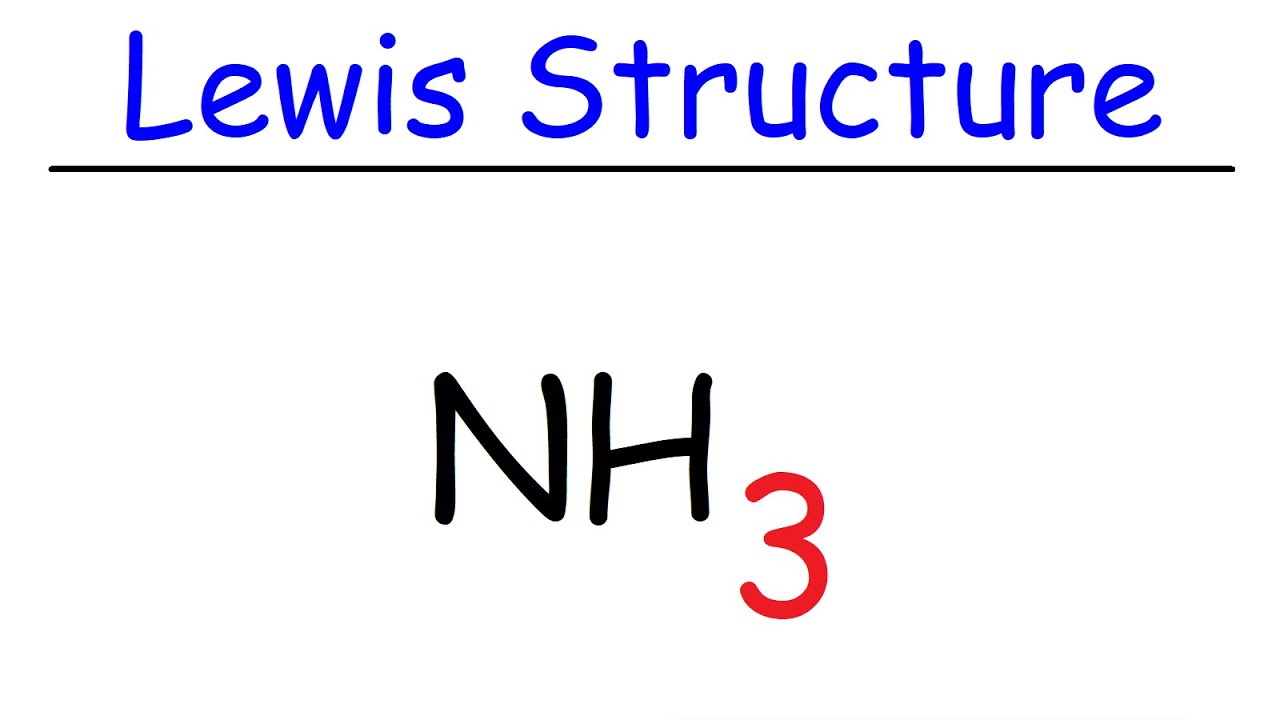
Nh3 Lewis Structure Ammonia Youtube
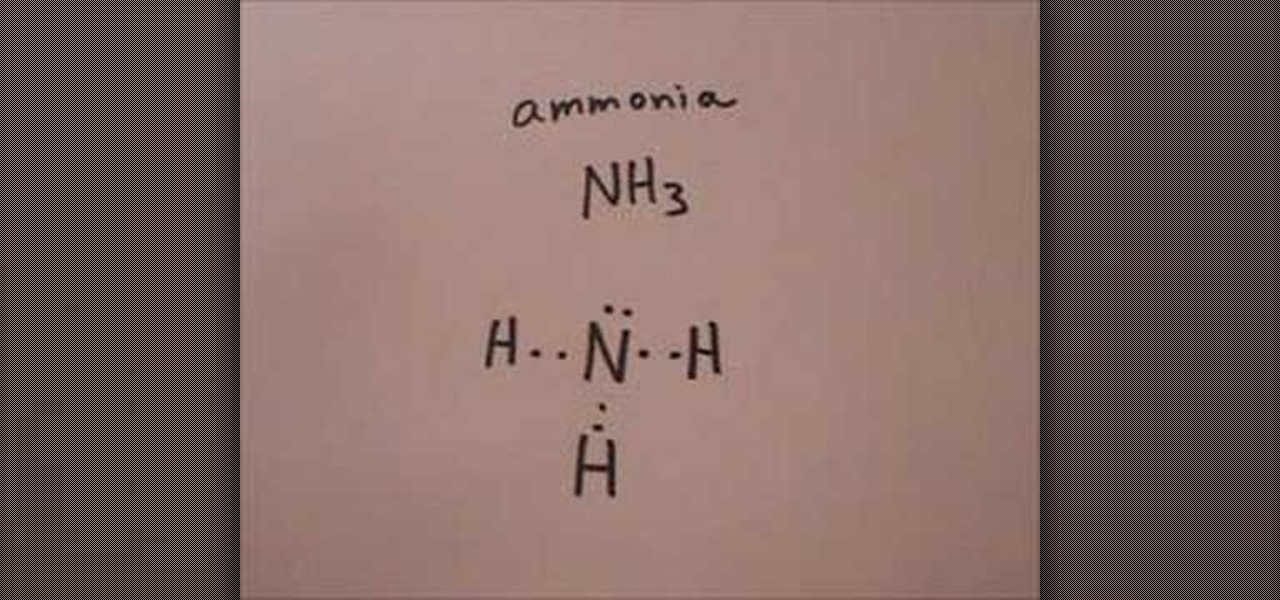
How To Draw The Lewis Structure For Ammonia Science Experiments Wonderhowto

Two Students Made The Lewis Dot Diagrams Of Nh3 The Diagrams Are As Shown Which Student Drew The Brainly Com

Nh3 Lewis Structure Geometry And Hybridization Techiescientist

Dot And Cross Structure For Nh3 Ammonia Youtube

Resonance Structures For Nh3 Ammonia Youtube
Lewis Electron Dot Structures Ck 12 Foundation

Nh3 Lewis Structure Ammonia Youtube

Calculating Nh3 Formal Charges Calculating Formal Charges For Nh3 Ammonia Youtube

Nh3 Ammonia Lewis Dot Structure Youtube
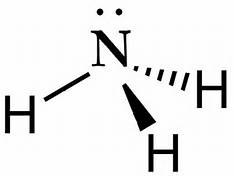
How Is Ammonia Represented By An Electron Dot Diagram Socratic


