Lewis Electron Dot Structure Of N2
The two letter Ns in the N 2 Lewis structure represent the nuclei centers of the nitrogen atoms. Firstly check out the atomic number of each atom from the Periodic Table.

Simple Method For Writing Lewis Structures For N2o3 Molecular Geometry Molecular Shapes Chemistry Help
In N2 Lewis structuretwo nitrogen atoms has shared six valence electrons and every nitrogen atom has one lone pairs In N2 Lewis.

Lewis electron dot structure of n2. Once we know how many valence electrons there are in Bari. For the N2 Lewis structure we have five valence electrons for Nitrogen--its in group 5 or 15 on the periodic table. In the middle you will found 3 pair making bond order 3.
We have two Nitrogens. Here is the electron dot structure for a single N atom. Lewis Dot Structure Of N2 n2 formula electron svg pixels wikimedia commons nominally kb n2 electron formula svg pixels wikimedia commons nominally kb n2 electron formula svg wikimedia commons pixels n2 electron formula svg pixels wikimedia commons nominally kb.
In order to come up with this answer you first need to know the number of valence electrons for Nitrogen. N2 Lewis Structure Answer. A common exception to this rule is the first row elements H and He.
In nitrogen gas structure there are 5 total electron pairs which were made by 25 electron pairs5 electrons for every nitrogen atom. For the N2 Lewis structure we have five valence electrons for Nitrogen--its in group 5 or 15 on the periodic table. Lewis Electron Dot Structure N2 What is the Lewis structure of N2.
That atoms form bonds to achieve eight electrons in their valence shell. N2 Lewis structure nitrogen electron dot structure is that type of structure where we show the total ten valence electrons of N2 as dots or dots and dashes -In Lewis structureit is common that a bonding pair of two electrons can be shown by dash - or dots but a lone pair of two electrons is shown by dots. Multiply those together we have a total of 10 valence electrons for the N2 Lewis structure.
Rules to Draw Lewis Structure. Each N is surrounded by two dots and three sticks or lines representing another 6 electrons in the N 2 triple bond. So each N is surrounded by 8 total valence electrons giving it an octet and making it stable.
In each nitrogen there will be 1 nonbonding electron making it a total 2 unbonding electron pairs. Multiply those together we have a total of 10 valence electrons for the N2 Lewis structure. The drawing of Lewis electron-dot structures is guided largely by the octet rule.
For many elements a full valence shell has an electron configuration of s2p6 or eight electrons. If the bond energy for the NN bond is 946 kJmol how much energy is needed to break all the bonds in 30 mol of nitrogen molecules. We have two Nitrogens.
Well put the two Nitrogens next to each other and then well put two valence electrons between them to form a chemical bond. For the Ba 2 structure use the periodic table to find the total number of valence electrons for B. What is the lewis dot structure for n2.
We have two Nitrogens. N2 Lewis Structure Molecular Geometry and Hybridization Lewis Dot Structure. A step-by-step explanation of how to draw the N2 Lewis Dot Structure Nitrogen Gas - Diatomic NitrogenFor the N2 structure use the periodic table to find t.
Since N is a member of the Group 5A based on the periodic table the number of electrons in its outermost shell must be 5. The nuclei contain the protons and neutrons which are the solid parts of the molecule. These two elements have n1 as their valence shell and so they.
The lewis electron-dot structure of n2 has ________ nonbonding electrons pairs ________ bonding electron pairs and a bond order of ________. Also for the structure to be correct it must follow the octet rule eight electrons. In N2 Lewis structuretwo nitrogen atoms has shared six valence electrons and every nitrogen atom has one lone pairs In N2 Lewis.
Socratic NF3 Lewis Structure How to Draw the Dot Structure for Bonding Basics Lewis Structure of N2O4 YouTube. We have a total of 10 valence. Draw the Lewis structures of all the molecules involved in the reaction.
The total number of valence electrons between the two N atoms is 10 e-. The Lewis structure indicates the atom and its position in the model of the molecule using its. Lewis Structure of N2 Nitrogen Gas - YouTube.
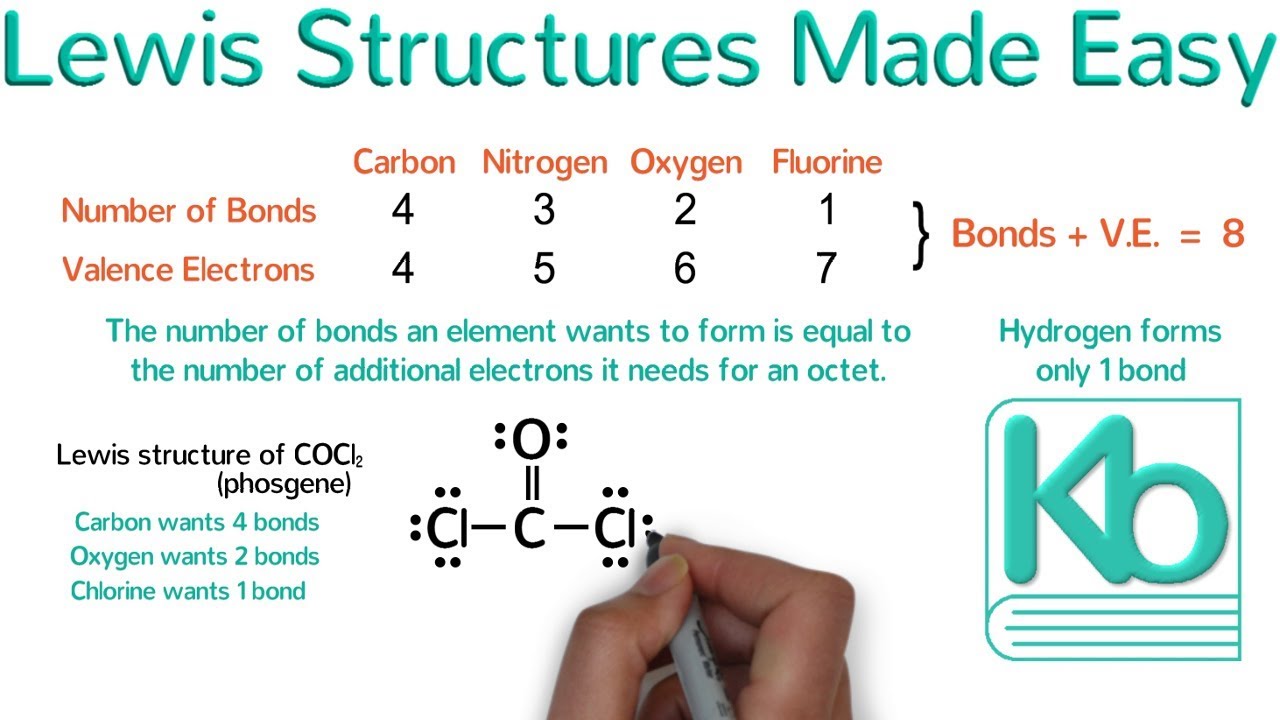
Lewis Structures Made Easy Examples And Tricks For Drawing Lewis Dot Diagrams Of Molecules Yo High School Chemistry Teaching Science Organic Chemistry Study

Lewis Dot Structure Electron Configuration Chemistry Lessons High School Chemistry

N2o5 Lewis Structure How To Draw The Lewis Structure For N2o5 Youtube Drawings Lewis Design

Xef2 Lewis Structure How To Draw The Lewis Structure For Xef2 In 2021 Lewis Molecules Math Equations

Drawing A Lewis Structure For Dinitrogen Monoxide Nitrous Oxide N2o Linked To Lewis Structures How Tos

Bonding In Ammonia Three Hydrogen Atoms Each Share One Electron And A Nitrogen Atom Shares Three Electrons Chemistry Classroom Science Revision Aqa

Is Nh2 Polar Or Non Polar Amide Ion In 2021 Nh 2 Molecules Electrons

Im Dunkeln Leuchten Lewis Dot Diagramme Senior Chemie Chemie Chemie D Chemie Dot Organic Chemistry Study Teaching Chemistry Chemistry Lessons

A Table Is Shown With Four Rows The Header Row Reads Metal Nonmetal And Ionic Compound The Second Row Shows Th Ionic Compound Ionic Bonding Chemistry

N3 Lewis Structure Azide Ion In 2021 Math Equations Lewis Molecules

Lewis Dot Structure For Hydrogen H Lewi Electron Configuration Chemistry Worksheets Chemical Equation

Electron Dot Structure Or Lewis Structure Electron Configuration Covalent Bonding Math Addition

Simple Method For Writing Lewis Structures For N2o3 Molecular Geometry Chemistry Help Molecular Shapes

Lewis Structures Made Easy Examples And Tricks For Drawing Lewis Dot Diagrams Of Molecules Yo High School Chemistry Teaching Science Organic Chemistry Study

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar



